
The role of endosonography-guided fine needle aspiration in preoperative restaging in the era of novel neoadjuvant therapies—a literature review
Introduction
Currently, chemoradiotherapy is the treatment of choice for stage III (IIIA-N2/IIIB-N3) non-small cell lung cancer (NSCLC). However, selected patients with N2/N3 disease, particularly those with a disease responding considerably to induction chemotherapy, radiotherapy, chemoradiotherapy, targeted therapy, and/or immunotherapy may be candidates for surgical resection and mediastinal lymph node dissection (1,2). Persistence of mediastinal metastases after induction therapy generally denotes poor surgical outcomes (3-5). In this regard, the importance of identifying successfully down-staged patients who can subsequently benefit from surgical resection is increasing. Restaging mediastinum accurately in lung cancer is critical as the disease stage is the main determinant of prognosis and guides for management options. However, how to restage mediastinum effectively in NSCLC patients has been a controversial issue.
The diagnostic performance of computed tomography (CT), positron emission tomography (PET) or PET/CT in restaging varies largely among different studies (6-8). Owing to their unsatisfactory sensitivities and specificities in mediastinal restaging, tissue sampling is required for determining the mediastinal lymph node status accurately.
Mediastinoscopy, an invasive surgical procedure, confirms or excludes N2 or N3 disease histologically in most patients with potentially operable NSCLC (9,10). However, remediastinoscopy is considered to be technically difficult owing to adhesions and fibrotic changes subsequent to the initial staging procedure and induction treatment (11,12). Consequently, it has lower accuracy (12,13) than primary mediastinoscopy (14). Furthermore, thoracoscopy and other surgical approaches are invasive, costly and may be challenging. Restaging by video-assisted thoracoscopic surgery (VATS), although feasible, is limited to one hemithorax because it requires single-lung ventilation. The sensitivity, specificity and negative predictive value of VATS for restaging after induction therapy were reported to be 67%, 100% and 73%, respectively by only one prospective multi-institutional trial. VATS restaged the mediastinum in 69% of patients but failed in 31% owing to the unmet pre-study feasibility endpoints in 38%, false-negative stations in 15%, necessity to abort the procedure due to pleural adhesions, tumor bulk, airway injury and inability to achieve atelectasis in 16% (15).
Conventional transbronchial needle aspiration (cTBNA) is a minimally invasive, safe, economical but a “blind” bronchoscopic technique that can histologically or cytologically determine the diagnosis and involvement of hilar and mediastinal lymph nodes in lung cancer. Initially introduced to medicine in 1949 but more widely used in clinical practice since 1978, cTBNA can be adequate in staging when the lymph nodes are larger than 1.5–2 cm and close to carina (paratracheal and subcarinal) in selected patients with a high pretest clinical probability of malignancy (16-19). Its accuracy in staging varies widely among pertinent studies and is shown to be significantly dependent on the prevalence of mediastinal involvement and operator skills. It has a high false-negative rate and thus, cannot be considered as a definitive mediastinal staging technique in routine practice (18,20,21). Although there is very limited data on the diagnostic performance of cTBNA in restaging (17,22), it is most likely that this technique cannot be definitive also in restaging lung cancer (17,23).
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and endoesophageal ultrasound-guided fine-needle aspiration (EUS-FNA) are minimally invasive endoscopic procedures used to detect metastases to mediastinal nodes. In the initial staging of lung cancer, both procedures are confirmed to provide accurate results (24-26). Although widely varying accuracies of EBUS-TBNA and EUS-FNA in the restaging of the mediastinum were reported by many studies with relatively small number of subjects (8,18,27), several pooled analyses of large pertinent data recently have shown reasonable diagnostic operating characteristics of both methods in mediastinal restaging after induction treatment for lung cancer (28,29).
This literature review will focus mainly on the role of endosonography (EBUS-TBNA, EUS-FNA, and combined EBUS/EUS-FNA) in preoperative restaging. For this purpose, a search was performed in PubMed for relevant studies, reviews and meta-analyses written in English in the last 2 decades on diagnostic performances of endosonography-guided fine needle aspiration and other methods in restaging stage III lung cancer after neoadjuvant therapy. I present the following article in accordance with the Narrative Review reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-21-104/rc).
Methods
For this literature review, an ethics committee approval was not required as it was performed to analyze already published studies, reviews and meta-analyses.
Between July 4, 2021 and November 14, 2021, a search was performed in Pubmed and Google for English articles published between 1998 and 2021 on diagnostic performances of endosonography-guided fine needle aspiration and other methods in mediastinal restaging of stage III lung cancer after neoadjuvant therapy. The following free text terms were used in the literature search: bronchoscopy, endoscopic ultrasound-guided fine needle aspiration, endosonography, EBUS, EUS, mediastinal restaging, and/or lung cancer.
A set of inclusion and exclusion criteria were used to select the most appropriate and reliable articles for this literature review.
Inclusion criteria
- Only published prospective or retrospective studies, reviews and meta-analyses in English.
- Only articles on EBUS-TBNA, EUS-FNA, combined EBUS/EUS-FNA, and other methods used for mediastinal restaging following neo-adjuvant therapy in stage III lung cancer.
Exclusion criteria
- Any unpublished online or printed data, study, review, etc.
- Articles published in a language other than English.
- Articles not providing sufficient information regarding diagnostic performance measures of EBUS-TBNA, EUS-FNA, combined EBUS/EUS-FNA and other mediastinal restaging methods (sensitivity, specificity, negative predictive, positive predictive value and/or accuracy, or true-positive, true-negative, false-positive and false negative values).
- Case reports, case series, conference abstracts, letters, editorials, and expert opinions.
The literature selection, data extraction and verification, disagreement resolution, and article quality assessment processes were conducted by one investigator (Semra Bilaceroglu) through the utilization of some of the methods reported previously (28,29). The strategy employed for the literature search is summarized in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 04.07.2021–14.11.2021 |
| Databases and other sources searched | PubMed, Google |
| Search terms used | Bronchoscopy, endoscopic ultrasound-guided fine needle aspiration, endosonography, EBUS, EUS, mediastinal restaging, lung cancer |
| Timeframe | 1998–2021 |
| Inclusion and exclusion criteria | Inclusion criteria: |
| 1. Only published studies, reviews and meta-analyses in English language | |
| 2. Only articles on diagnostic performances of endosonography- guided fine needle aspiration and other methods in restaging stage III lung cancer after neoadjuvant therapy | |
| Exclusion criteria: | |
| 1. Any unpublished relevant data | |
| 2. Relevant publications in languages other than English | |
| 3. Articles other than studies, reviews and meta-analyses | |
| 4. Articles with insufficient data on the assessed diagnostic performance measures | |
| Selection process | The literature selection, data extraction and verification, disagreement resolution, and article quality assessment processes were conducted by one investigator (Semra Bilaceroglu) through the utilization of some of the methods reported previously (28,29) |
Endosonography-guided needle aspiration in staging and restaging
Diagnostic performance
Mediastinal lymph node staging of lung cancer can be performed using invasive or noninvasive methods. The most commonly used noninvasive methods, chest CT and PET or PET/CT, are safe but limited regarding diagnostic performance with low sensitivity and specificity. In restaging mediastinum, CT has an accuracy of only 58–60% (6,12). PET/CT has a higher sensitivity (73–92%) but a low specificity (62–89%) due to false-positive results (6,8). Furthermore, the pooled specificity of PET/CT is lower (61%) for malignancy in regions with high prevalence of endemic pulmonary infections compared with those that are nonendemic (30). Owing to false-positive results due to these infections, confirming metastasis by only imaging methods is not reliable; histological confirmation is required.
Although mediastinoscopy has been considered to be the gold standard with a sensitivity of 80–91% in staging mediastinum, the repeated procedure has several drawbacks compared with the initial one: a 2% major morbidity risk, a 0.08% mortality risk, substantial cost (6,13,31,32), technical difficulty, lower accuracy due to a high false-negative rate (22%), and a low and variable sensitivity (29–73%) (6,13,33). Transcervical extended mediastinal lymphadenectomy has a high sensitivity (97%) in mediastinal restaging but its mortality and morbidity rates are relatively high (0.3% and 6.4%, respectively), and this procedure is not widely practiced (34).
In clinical N2/N3 disease with a high disease prevalence of 75–81%, cTBNA has a sensitivity of 76–78% and a false-negative rate of 28–29% (21,35,36) in diagnosis and staging. The utility of cTBNA in routine mediastinal staging is compromised by this high false-negative rate. The sensitivity of cTBNA depends on the prevalence of mediastinal lymph node metastases and lymph node size. Its sensitivity tends to decrease with lower prevalence of mediastinal metastases (35) and smaller node size (<15–20 mm short axis on CT scan) (17,18,21). In only one study on diagnostic performance of cTBNA in restaging, correct restaging could be done by this method in 71% of 14 patients and 81% of 17 lymph nodes (22). In some of the studies on staging by cTBNA, only small number of patients were restaged using this procedure (17). Thus, owing to the inadequate evidence on its restaging performance and inferences drawn from its variable and low staging performance that has been studied widely, cTBNA cannot be used as a definitive approach in restaging lung cancer.
As minimally invasive procedures, EBUS-TBNA and EUS-FNA have become standard methods for staging mediastinal nodes in lung cancer. The Executive Summary of the American College of Chest Physicians (ACCP) Evidence-based Clinical Practice Guidelines on Diagnosis and Management of Lung Cancer (3rd edition) (26) and a more recently issued guidelines by the European Society of Gastrointestinal Endoscopy in collaboration with the European Respiratory and Thoracic Surgeons Societies (37) have recommended endosonography (EBUS-TBNA, EUS-FNA, or combined EBUS/EUS-FNA) over surgery as a best first test in staging NSCLC. The latter guidelines have further recommended combined EBUS/EUS-FNA over either procedure alone in staging. The use of EBUS-TBNA, EUS-FNA, or combined EBUS/EUS-FNA in restaging mediastinum after neoadjuvant therapy have also been suggested with a grade C recommendation (37).
EBUS-TBNA has been corroborated to have a high yield (89–98%) in diagnosing and staging lung cancer (38-41). The sensitivity of EUS-FNA for initial staging of lung cancer ranges between 45% and 80% (42-44). In a recent systematic review including 558 patients from 10 studies, the pooled sensitivity of EBUS-TBNA in lung cancer restaging was found to be 65%, and that of EUS-FNA to be 73% while pooled sensitivities of combined EBUS/EUS-FNA and overall endosonography-guided FNA were 67% and 70%, respectively. In this review, the pooled specificity was 100% for overall endosonography-guided FNA, 99% for each of EBUS-TBNA and EUS-FNA, and 96% for combined EBUS/EUS-FNA. The sensitivity (66%) and specificity (100%) of endosonography-guided FNA after chemotherapy alone were similar to those after chemoradiotherapy (77% and 99%, respectively) (28). Another recent systematic review and meta-analysis including 574 patients from 10 studies demonstrated that the pooled sensitivity, specificity, diagnostic odds ratio, and positive and negative likelihood ratios of endosonography (EBUS, EUS and combined EBUS/EUS)-guided FNA were 67% (40–89%), 99% (91–99%), 157, 52.0, and 0.33, respectively, with an area under the receiver-operating characteristic curve of 0.93 (29). Thus, the diagnostic performance of EBUS-TBNA, EUS-FNA, or their combination is confirmed to be lower in restaging than that in the initial staging. The diagnostic performances of endosonography-guided procedures versus those of imaging, cTBNA, and surgical methods in mediastinal restaging of lung cancer are given in Table 2.
Table 2
| Sensitivity | Specificity | NPV | PPV | Accuracy | |
|---|---|---|---|---|---|
| CT | 41–59% (20–91%) | 62–75% (50–97%) | 47–56% (38–97%) | 43–70% (39–92% ) | 58–67% (37–92%) |
| PET/CT | 77–89% (54–92%) | 61–80% (48–93%) | 71–87% (71–100%) | 36–75% (33–93%) | 72–87% (67–94%) |
| cTBNA | 71% | – | – | – | – |
| EBUS-TBNA | 64–70% (33–82%) | 85–99% (78–100%) | 55–76% (20–82%) | 91–96% (80–100%) | 74–81% (64–92%) |
| EUS-FNA | 61–75% (44–92%) | 96–99% (90–100%) | 71–82% (58–91%) | 97–100% (83–100%) | 72–81% (60–92%) |
| EBUS/EUS-FNA | 67–70% (53–79%) | 94–98% (86–99%) | 73–76% (61–83%) | 95% (83–99%) | 81–83% (73–87%) |
| Overall endosonography-FNA | 67–70% (40–89%) | 99–100% (87–100%) | 76–81% (20–93%) | 96–100% (87–100%) | 91–93% (88–95%) |
| Re-mediastinoscopy | 61–74% (29–90%) | 100% | 73–79% (52–86%) | 100% | 84–88% (60–94%) |
| TEMLA | 95–100% | 100% | 97–100% | 100% | 98–99% |
| VATS | 62–67% (47–83%) | 100% | 73% (56–86%) | 100% | 63–72% (40–82%) |
cTBNA, conventional transbronchial needle aspiration; NPV, negative predictive value; PPV, positive predictive value; CT, computerized tomography; PET/CT, positron emission tomography/computerized tomography; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; EUS-FNA, endoesophageal ultrasound-guided fine needle aspiration; EBUS/EUS-FNA, combined endobronchial and endoesophageal ultrasound-guided fine needle aspiration; overall endosonography-FNA, overall endosonography-guided fine needle aspiration; TEMLA, transcervical extended mediastinal lymphadenectomy; VATS, video-assisted thoracoscopic surgery.
Safety
Endosonographic procedures rarely cause complications. A systematic review on adverse events related to endosonography for mediastinal, hilar, or primary lung tumor analysis reported a 0.14% rate of serious adverse events in 16,181 patients: 0.05% with EBUS, 0.3% with EUS (45). Another systematic review including 13 studies (1,536 patients) also showed the safety of EBUS-TBNA in lung cancer (46): no complications in 11 studies, no major complication in 1 study, and rare side effects (e.g., cough) in 1 study. A third systematic review confirmed that severe complications (pneumothorax and lymph node abscess) occurred in 0.3% of the patients undergoing combined EUS-FNA and EBUS-TBNA for mediastinal staging in lung cancer (47). Furthermore, a nationwide survey on complications associated with EBUS-TBNA conducted by the Japan Society for Respiratory Endoscopy demonstrated a complication rate of 1.23% (95% CI: 0.97–1.48%) and a mortality rate of 0.01% within 7,345 procedures in 210 facilities. The death, reported in a single patient, was due to cerebral infarction after withdrawal of antiplatelet drugs that were replaced by heparin. Hemorrhage (55%) and infection (16%) were the most frequent of all complications (48).
In restaging lung cancer too, endosonographic procedures have been confirmed to be safe with comparable types and rates of complications that were not severe (27-29,34).
Limitations and challenges
The main limitation of endosonography by EBUS or EUS is that some of the mediastinal lymph node stations such as 5 (subaortic) and 6 (para-aortic) cannot be accessed by EBUS or EUS. Nevertheless, minimally invasive mediastinal staging can be accomplished to a near-complete level by combining EBUS-TBNA and EUS-FNA (49,50).
EBUS-TBNA has been demonstrated to have a higher diagnostic yield than cervical mediastinoscopy by multiple studies (51,52) as it can access lymph node stations that cervical mediastinoscopy cannot. In a meta-analysis aiming to assess the diagnostic yield of combined EBUS-TBNA and EUS-FNA in NSCLC staging (47), the sensitivity, specificity and negative likelihood ratio were 86%, 100% and 0.15, respectively. The combined approach had a significantly higher sensitivity than that of each strategy alone (47). EUS-FNA and mediastinoscopy can be combined in the diagnosis and staging of lung cancer if EBUS-TBNA is not available (18).
Of the two studies in which combined endosonography was performed for restaging NSCLC (34,53), only one has reported the sensitivity (67%), specificity (96%) and accuracy (81%) of the combined procedure in restaging (53). However, the perfect specificity but defective sensitivity of EBUS-TBNA, EUS-FNA or the combination of both in restaging has been proved convincingly by the two recent systematic reviews mentioned above (28,29).
Formation of areas of necrosis, inflammation and fibrosis in the metastatic lymph nodes due to induction treatment (chemotherapy, radiotherapy, chemoradiotherapy, targeted therapy and/or immunotherapy) (2,53,54) and fibrotic changes secondary to the initial staging procedure (11,12) can most likely be the causes of defective sensitivity. Less cellular material even in the properly obtained samples from these lymph nodes may be challenging in histologic analysis. Focally located malignant cells within the treated metastatic lymph nodes or in dense extracellular matrix may partially explain why false-negative samples with no malignant cells occur although successful aspiration of adequate lymph node tissue is accomplished. Furthermore, necrosis within the aspirated sample challenges pathologic interpretation (27,28). Other possible reasons for the decreased sensitivity can be shrunken nodes with smaller size as well as difficulties in accessing some nodes and in differentiating metastasis from adhesions/degenerative changes consequent to induction therapy (29).
EUS-FNA using the EBUS scope
Endoesophageal ultrasound guided fine-needle aspiration using the EBUS scope (EUS-B-FNA) can be performed by a pulmonologist in the same session with EBUS-TBNA. With EUS-B-FNA, the procedural cost is not increased considerably. After EBUS-TBNA procedure, the EBUS scope is pulled from the airways and inserted into the esophagus for EUS-FNA (37,55).
EUS-B-FNA has also been used to sample left adrenal nodules as part of lung cancer staging. The EBUS scope advanced to the stomach is used to identify and sample the left adrenal nodule under real-time EUS-B-FNA. This procedure is suggested to lower the cost in selected patients (56) although no data on cost-effectiveness have been available yet.
Limitations of current data and factors affecting endosonographic restaging performance
In the studies used for the pooled data analyses most important limitations are lack of standardization in diagnostic testing and treatment: variations in the time interval between the completion of neoadjuvant therapy and restaging by CT, PET/CT, endosonography or surgical procedures [optimally about 1 month (57) but varying from 2 to 12 weeks (8,27,57,58)], not using PET/CT routinely, methodologic heterogeneity regarding the technical aspects of endosonography and handling/processing specimens, variations in strategies for primary pathologic staging and induction treatment, use of various therapeutic agents (chemotherapy, targeted therapy and/or immunotherapy) and numbers of treatment cycles, and the use of radiation in addition to chemotherapy, targeted therapy and/or immunotherapy in some patients (28,29).
In most studies, owing to ethical issues, positive results from endosonographic restaging are not confirmed by surgery as the reference standard. Furthermore, the majority of the studies analyzed in the pooled data analyses and systematic reviews are small studies from single centers and specialized endoscopy or thoracic surgery departments. Corroboration of these results is required by larger, world-wide and multi-center studies (28).
Widely varying prevalence of N2 disease (19–94%) in the studies analyzed could have an effect on the sensitivity and accuracy since a proportional increase in sensitivity occurs with increasing N2 prevalence (27,29,58).
All neoadjuvant therapies, old or novel, also have the potential to affect the accuracy of restaging for lung cancer owing to the pathologic changes shown in resected tissues after neoadjuvant treatments: fibroelastotic changes, proliferative fibrosis, necrosis, vasculitis, vascular thickening and obliteration, neovascularization, cytologic atypia of tumor cells, lymphocytosis in tumor tissue and chronic inflammatory cell infiltrates (2,54,59,60).
More studies on the ideal restaging procedures are needed. In the studies included in the pooled data analyses for restaging, description of the lymph node histopathologic structure is not available. This description may be helpful in sampling. Further investigations on how the malignant cells are distributed in the treated lymph nodes can provide significant information. Demonstration of central, peripheral or focally scattered distribution of malignant cells in the lymph node may help the endoscopists in choosing the location for needle insertion during EBUS-TBNA or EUS-FNA. Consequently, this may have an impact on the size of the sampling needle, how many locations to sample in the lymph node, number of needle passes, and use of rapid onsite evaluation (ROSE) to increase the yield (28,29,61).
As the sensitivity of endosonography-guided needle aspiration varies widely (44–72%) for restaging in the studies reporting an average of three needle passes along with ROSE (27,62,63), whether the sensitivity of endosonographic needle aspiration can be improved by multiple passes together with ROSE is currently uncertain. Similar uncertainty exists for needle size. Owing to the use of a 22-G needle in most of the studies included in the pooled data analyses, the effect of needle size could not be explored. In theory, larger (19-gauge) needles are expected to obtain more diagnostic and adequate histologic and cytologic samples than smaller needles. However, in real-world practice larger needles have not provided significantly higher but rather comparable diagnostic performance in EBUS-TBNA whether it is 21-gauge versus 22-gauge (64,65), 22-gauge versus 25-gauge (66), or 19-gauge versus 21- or 22-gauge (67,68). More frequently obtained bloody cytologic specimens and inadequate specimens due to more difficulty in puncturing the tissue with a 19-gauge needle might have caused its lower than expected performance (68).
Furthermore, access to certain lymph nodes by EBUS and EUS are limited or not possible: e.g., stations 8 and 9 by EBUS and station 4R by EUS. Thus, the sensitivity and specificity of EBUS or EUS apply only to the locations accessible by either method. Indeed, these two approaches cannot be compared as each is a stand-alone staging technique but can be complementary to the other. Prospective studies are needed to answer all of the above-mentioned issues and how to increase the diagnostic yield of endosonography in restaging lung cancer (26,29,37).
Clinical implications
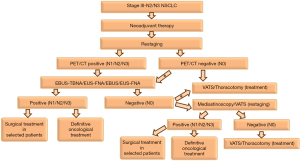
In clinical practice, endosonographic procedures are ideal in the initial evaluation for restaging of NSCLC owing to their high specificity and positive likelihood ratios (69). Rarely reported false-positive results (1.9–5%) are not due to the method but the misidentification of the pulmonary tumor as a lymph node and how appropriately the procedure was performed by the endoscopist (53,61). As a reliable rule-in test, a positive result obtained by endosonography-guided FNA obviates further evaluation, particularly surgical procedures in approximately 67% of the patients (28,29,70). Combining EBUS and EUS appears to provide higher yields in mediastinal restaging as they are complementary to each other (53). A negative result by endosonographic restaging requires confirmation by a surgical procedure. However, in the pertinent literature there has been no direct comparison of endosonography and mediastinoscopy or other surgical methods in mediastinal restaging. A clinically reasonable pathway for mediastinal restaging should be implemented and tailored for each individual patient through a discussion in a multidisciplinary team consisting pulmonologists, thoracic surgeons, medical oncologists and radiation oncologists (28,29,70) (Figure 1). Furthermore, the observations from the pooled data analyses and meta-analyses should be evidenced by randomized controlled studies.
Conclusions
Endosonography-guided needle aspiration has a reasonable sensitivity and a high specificity in the mediastinal restaging of NSCLC. Moreover, it is safe with low complication rates. Thus, it is promising as an initial rule-in investigation in restaging the mediastinum following neoadjuvant therapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Surgical Journal for the series “Impact of Novel Neoadjuvant Treatment on Surgery Outcomes in Lung Cancer”. The article has undergone external peer review.
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-21-104/rc
Peer Review File: Available at https://asj.amegroups.com/article/view/10.21037/asj-21-104/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-21-104/coif). The series “Impact of Novel Neoadjuvant Treatment on Surgery Outcomes in Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The author served as the unpaid Guest Editor of the series. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Allaeys T, Berzenji L, Van Schil PE. Surgery after Induction Targeted Therapy and Immunotherapy for Lung Cancer. Cancers (Basel) 2021;13:2603. [Crossref] [PubMed]
- Sawabata N, Keller SM, Matsumura A, et al. The impact of residual multi-level N2 disease after induction therapy for non-small cell lung cancer. Lung Cancer 2003;42:69-77. [Crossref] [PubMed]
- Bueno R, Richards WG, Swanson SJ, et al. Nodal stage after induction therapy for stage IIIA lung cancer determines patient survival. Ann Thorac Surg 2000;70:1826-31. [Crossref] [PubMed]
- Decaluwé H, De Leyn P, Vansteenkiste J, et al. Surgical multimodality treatment for baseline resectable stage IIIA-N2 non-small cell lung cancer. Degree of mediastinal lymph node involvement and impact on survival. Eur J Cardiothorac Surg 2009;36:433-9. [Crossref] [PubMed]
- De Leyn P, Stroobants S, De Wever W, et al. Prospective comparative study of integrated positron emission tomography-computed tomography scan compared with remediastinoscopy in the assessment of residual mediastinal lymph node disease after induction chemotherapy for mediastinoscopy-proven stage IIIA-N2 Non-small-cell lung cancer: a Leuven Lung Cancer Group Study. J Clin Oncol 2006;24:3333-9. [Crossref] [PubMed]
- Pöttgen C, Levegrün S, Theegarten D, et al. Value of 18F-fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography in non-small-cell lung cancer for prediction of pathologic response and times to relapse after neoadjuvant chemoradiotherapy. Clin Cancer Res 2006;12:97-106. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Ojha B. Restaging patients with N2 (stage IIIa) non-small cell lung cancer after neoadjuvant chemoradiotherapy: a prospective study. J Thorac Cardiovasc Surg 2006;131:1229-35. [Crossref] [PubMed]
- Hammoud ZT, Anderson RC, Meyers BF, et al. The current role of mediastinoscopy in the evaluation of thoracic disease. J Thorac Cardiovasc Surg 1999;118:894-9. [Crossref] [PubMed]
- Porte H, Roumilhac D, Eraldi L, et al. The role of mediastinoscopy in the diagnosis of mediastinal lymphadenopathy. Eur J Cardiothorac Surg 1998;13:196-9. [Crossref] [PubMed]
- Pitz CC, Maas KW, Van Swieten HA, et al. Surgery as part of combined modality treatment in stage IIIB non-small cell lung cancer. Ann Thorac Surg 2002;74:164-9. [Crossref] [PubMed]
- Mateu-Navarro M, Rami-Porta R, Bastus-Piulats R, et al. Remediastinoscopy after induction chemotherapy in non-small cell lung cancer. Ann Thorac Surg 2000;70:391-5. [Crossref] [PubMed]
- Van Schil P, van der Schoot J, Poniewierski J, et al. Remediastinoscopy after neoadjuvant therapy for non-small cell lung cancer. Lung Cancer 2002;37:281-5. [Crossref] [PubMed]
- Toloza EM, Harpole L, Detterbeck F, et al. Invasive staging of non-small cell lung cancer: a review of the current evidence. Chest 2003;123:157S-66S. [Crossref] [PubMed]
- Jaklitsch MT, Gu L, Demmy T, et al. Prospective phase II trial of preresection thoracoscopic mediastinal restaging after neoadjuvant therapy for IIIA (N2) non-small cell lung cancer: results of CALGB Protocol 39803. J Thorac Cardiovasc Surg 2013;146:9-16. [Crossref] [PubMed]
- Yang H, Zhang Y, Wang KP, et al. Transbronchial needle aspiration: development history, current status and future perspective. J Thorac Dis 2015;7:S279-86. [PubMed]
- Fiorelli A, Santoriello C, Di Natale D, et al. In the era of ultrasound technology, could conventional trans-bronchial needle aspiration still play a role in lung cancer mediastinal staging? J Thorac Dis 2017;9:S386-94. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Medford AR, Agrawal S, Free CM, et al. A prospective study of conventional transbronchial needle aspiration: performance and cost utility. Respiration 2010;79:482-9. [Crossref] [PubMed]
- Leiro-Fernández V, Fernández-Villar A. Mediastinal staging for non-small cell lung cancer. Transl Lung Cancer Res 2021;10:496-505. [Crossref] [PubMed]
- Medford AR, Bennett JA, Free CM, et al. Mediastinal staging procedures in lung cancer: EBUS, TBNA and mediastinoscopy. Curr Opin Pulm Med 2009;15:334-42. [Crossref] [PubMed]
- Kunst PW, Lee P, Paul MA, et al. Restaging of mediastinal nodes with transbronchial needle aspiration after induction chemoradiation for locally advanced non-small cell lung cancer. J Thorac Oncol 2007;2:912-5. [Crossref] [PubMed]
- Khoo KL. Mediastinal re-staging of non small-cell lung cancer. Thorac Cancer 2012;3:145-9. [Crossref] [PubMed]
- Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis. Chest 2007;131:539-48. [Crossref] [PubMed]
- Adams K, Shah PL, Edmonds L, et al. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 2009;64:757-62. [Crossref] [PubMed]
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S.
- Nasir BS, Bryant AS, Minnich DJ, et al. The efficacy of restaging endobronchial ultrasound in patients with non-small cell lung cancer after preoperative therapy. Ann Thorac Surg 2014;98:1008-12. [Crossref] [PubMed]
- Jiang L, Huang W, Liu J, et al. Endosonography with lymph node sampling for restaging the mediastinum in lung cancer: A systematic review and pooled data analysis. J Thorac Cardiovasc Surg 2020;159:1099-1108.e5. [Crossref] [PubMed]
- Muthu V, Sehgal IS, Dhooria S, et al. Efficacy of Endosonographic Procedures in Mediastinal Restaging of Lung Cancer After Neoadjuvant Therapy: A Systematic Review and Diagnostic Accuracy Meta-Analysis. Chest 2018;154:99-109. [Crossref] [PubMed]
- Deppen SA, Blume JD, Kensinger CD, et al. Accuracy of FDG-PET to diagnose lung cancer in areas with infectious lung disease: a meta-analysis. JAMA 2014;312:1227-36. [Crossref] [PubMed]
- Harewood GC, Wiersema MJ, Edell ES, et al. Cost-minimization analysis of alternative diagnostic approaches in a modeled patient with non-small cell lung cancer and subcarinal lymphadenopathy. Mayo Clin Proc 2002;77:155-64. [Crossref] [PubMed]
- Aabakken L, Silvestri GA, Hawes R, et al. Cost-efficacy of endoscopic ultrasonography with fine-needle aspiration vs. mediastinotomy in patients with lung cancer and suspected mediastinal adenopathy. Endoscopy 1999;31:707-11. [Crossref] [PubMed]
- Marra A, Hillejan L, Fechner S, et al. Remediastinoscopy in restaging of lung cancer after induction therapy. J Thorac Cardiovasc Surg 2008;135:843-9. [Crossref] [PubMed]
- Zielinski M, Szlubowski A, Kołodziej M, et al. Comparison of endobronchial ultrasound and/or endoesophageal ultrasound with transcervical extended mediastinal lymphadenectomy for staging and restaging of non-small-cell lung cancer. J Thorac Oncol 2013;8:630-6. [Crossref] [PubMed]
- Holty JE, Kuschner WG, Gould MK. Accuracy of transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: a meta-analysis. Thorax 2005;60:949-55. [Crossref] [PubMed]
- Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:202S-20S.
- Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy 2015;47:c1. [Crossref] [PubMed]
- Yasufuku K, Chiyo M, Koh E, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer 2005;50:347-54. [Crossref] [PubMed]
- Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004;126:122-8. [Crossref] [PubMed]
- Yasufuku K, Nakajima T, Motoori K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest 2006;130:710-8. [Crossref] [PubMed]
- Lee JE, Kim HY, Lim KY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lung cancer. Lung Cancer 2010;70:51-6. [Crossref] [PubMed]
- Vilmann P, Krasnik M, Larsen SS, et al. Transesophageal endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy: a combined approach in the evaluation of mediastinal lesions. Endoscopy 2005;37:833-9. [Crossref] [PubMed]
- Wallace MB, Pascual JM, Raimondo M, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA 2008;299:540-6. [Crossref] [PubMed]
- Szlubowski A, Zieliński M, Soja J, et al. A combined approach of endobronchial and endoscopic ultrasound-guided needle aspiration in the radiologically normal mediastinum in non-small-cell lung cancer staging--a prospective trial. Eur J Cardiothorac Surg 2010;37:1175-9. [Crossref] [PubMed]
- von Bartheld MB, van Breda A, Annema JT. Complication rate of endosonography (endobronchial and endoscopic ultrasound): a systematic review. Respiration 2014;87:343-51. [Crossref] [PubMed]
- Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J 2009;33:1156-64. [Crossref] [PubMed]
- Zhang R, Ying K, Shi L, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer: a meta-analysis. Eur J Cancer 2013;49:1860-7. [Crossref] [PubMed]
- Asano F, Aoe M, Ohsaki Y, et al. Complications associated with endobronchial ultrasound-guided transbronchial needle aspiration: a nationwide survey by the Japan Society for Respiratory Endoscopy. Respir Res 2013;14:50. [Crossref] [PubMed]
- Sheski FD, Mathur PN. Endobronchial ultrasound. Chest 2008;133:264-70. [Crossref] [PubMed]
- Vilmann P, Puri R. The complete ''medical'' mediastinoscopy (EUS-FNA + EBUS-TBNA). Minerva Med 2007;98:331-8. [PubMed]
- Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304:2245-52. [Crossref] [PubMed]
- Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008;3:577-82. [Crossref] [PubMed]
- Szlubowski A, Zieliński M, Soja J, et al. Accurate and safe mediastinal restaging by combined endobronchial and endoscopic ultrasound-guided needle aspiration performed by single ultrasound bronchoscope. Eur J Cardiothorac Surg 2014;46:262-6. [Crossref] [PubMed]
- Travis WD, Dacic S, Wistuba I, et al. IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy. J Thorac Oncol 2020;15:709-40. [Crossref] [PubMed]
- Kang HJ, Hwangbo B, Lee GK, et al. EBUS-centred versus EUS-centred mediastinal staging in lung cancer: a randomised controlled trial. Thorax 2014;69:261-8. [Crossref] [PubMed]
- Crombag LMMJ, Szlubowski A, Stigt JA, et al. EUS-B-FNA vs conventional EUS-FNA for left adrenal gland analysis in lung cancer patients. Lung Cancer 2017;108:38-44. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. When is it best to repeat a 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography scan on patients with non-small cell lung cancer who have received neoadjuvant chemoradiotherapy? Ann Thorac Surg 2007;84:1092-7. [Crossref] [PubMed]
- Herth FJ, Annema JT, Eberhardt R, et al. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol 2008;26:3346-50. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]
- Xie H, Shi X, Wang G. Neoadjuvant immunotherapy for resectable non-small cell lung cancer. Am J Cancer Res 2021;11:2521-36. [PubMed]
- Szlubowski A, Herth FJ, Soja J, et al. Endobronchial ultrasound-guided needle aspiration in non-small-cell lung cancer restaging verified by the transcervical bilateral extended mediastinal lymphadenectomy--a prospective study. Eur J Cardiothorac Surg 2010;37:1180-4. [Crossref] [PubMed]
- von Bartheld MB, Versteegh MI, Braun J, et al. Transesophageal ultrasound-guided fine-needle aspiration for the mediastinal restaging of non-small cell lung cancer. J Thorac Oncol 2011;6:1510-5. [Crossref] [PubMed]
- Varadarajulu S, Eloubeidi M. Can endoscopic ultrasonography-guided fine-needle aspiration predict response to chemoradiation in non-small cell lung cancer? A pilot study. Respiration 2006;73:213-20. [Crossref] [PubMed]
- Muthu V, Gupta N, Dhooria S, et al. A Prospective, Randomized, Double-Blind Trial Comparing the Diagnostic Yield of 21- and 22-Gauge Aspiration Needles for Performing Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in Sarcoidosis. Chest 2016;149:1111-3. [Crossref] [PubMed]
- Giri S, Pathak R, Yarlagadda V, et al. Meta-analysis of 21- versus 22-G aspiration needle during endobronchial ultrasound-guided transbronchial needle aspiration. J Bronchology Interv Pulmonol 2015;22:107-13. [Crossref] [PubMed]
- Yang L, Gu Y, Wang H, et al. Novel ProCore 25-gauge needle for endobronchial ultrasound-guided transbronchial needle aspiration reduces the puncture time and frequency, with comparable diagnostic rate for mediastinal and hilar lymphadenopathy. Thorac Cancer 2020;11:748-53. [Crossref] [PubMed]
- Yu Lee-Mateus A, Garcia-Saucedo JC, Abia-Trujillo D, et al. Comparing diagnostic sensitivity of different needle sizes for lymph nodes suspected of lung cancer in endobronchial ultrasound transbronchial needle aspiration: Systematic review and meta-analysis. Clin Respir J 2021;15:1328-36. [Crossref] [PubMed]
- Chaddha U, Ronaghi R, Elatre W, et al. Comparison of Sample Adequacy and Diagnostic Yield of 19- and 22-G EBUS-TBNA Needles. J Bronchology Interv Pulmonol 2018;25:264-8. [Crossref] [PubMed]
- Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ 2004;329:168-9. [Crossref] [PubMed]
- Hiebinger A, Öfner-Velano D, Bodner J. VATS-lymph node dissection, staging and restaging in advanced malignancy/the Munich experience. Video-assist Thorac Surg 2018;3:29. [Crossref]
Cite this article as: Bilaçeroğlu S. The role of endosonography-guided fine needle aspiration in preoperative restaging in the era of novel neoadjuvant therapies—a literature review. AME Surg J 2022;2:36.


