
Minimally invasive retroperitoneal necrosectomy: how do we do it?
Introduction
Acute pancreatitis (AP) is an inflammatory condition that includes local and systemic manifestations. Between 70–80% of AP is classified as mild. The remaining 20–30%, more aggressive with a higher mortality rate (around 15%), is classified as moderate-severe (1), mainly related to necrotizing pancreatitis (2). All the (peri)pancreatic collections are susceptible to being infected, worsening the prognosis and duplicating the mortality risk (3).
In 2010, the PANTER trial compared the minimally invasive “step-up approach” with the standard treatment for the infected pancreatic necrosis, the open necrosectomy. The step-up approach was developed as an alternative to the open necrosectomy, to reduce major complications and mortality rates of the interventions for the infected pancreatic necrosis. Major complications, such as new-onset organ failure were present in 40% of patients who underwent open necrosectomy compared with 12% of patients assigned to the step-up approach. It is important to mention that the mortality between groups was no different and long-term outcomes were not evaluated (4). Today, it is acknowledged that, whenever possible, surgical major interventions should be delayed as long as possible. The behavior towards necrotizing pancreatitis relies on the clinical condition of the patient, the location, the availability of equipment, and the experience of the surgical team (5,6) (Figure 1). This manuscript aims to describe a minimally invasive therapeutic option for patients with necrotizing pancreatitis as well as some tips, tricks, and risks. Indications and contraindications are listed below (Table 1).
Table 1
| Indications | Contraindications |
|---|---|
| Multiple collections | Retroperitoneal access not possible |
| Retroperitoneal access | Presence of gastrointestinal fistula |
| Large-volume | High-risk organ injury (i.e., colon, spleen, kidney) |
| High solid debris component | General anesthesia risk |
| Extension to the lesser sac and/or paracolic gutter |
Patient’s workup
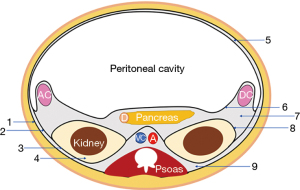
Most infected necrotizing pancreatitis progress within the second and fourth week, posterior to the initial inflammatory phase (1). The presence of gas within peripancreatic collections is highly sensitive and specific for infection. The contrast-enhanced computed tomography (CECT) allows the identification, localization, and quantification of the necrotic collections (Figure 1).
All candidates for laparoscopic retroperitoneal necrosectomy should be receiving supportive care, broad-spectrum antibiotic, and an unsuccessful percutaneous drainage background (1,4,8). To decrease the complications and mortality rates, the necrosectomy should be at least delayed until the fourth week since the onset of symptoms (8). The equipment preference card is shown below (Table 2).
Table 2
| Hydrophilic coated 0.035 guidewire |
| Two 10-mm trocars |
| One 5-mm trocar |
| Angled 30° laparoscope 10 mm |
| Atraumatic grasping and dissection instruments |
| Ultrasonic scalpel or bipolar coagulation device |
| Irrigation/suction handpiece |
| Silicone fenestrated 10–19 Fr drain (irrigation, more than one if required) |
| Silicone fenestrated 19–28 Fr drain (suction, more than one if required) |
| Hydrogen peroxide solution (2 liters) |
| Sterile 0.9% saline solution (5–8 liters) |
| Absorbable hemostatic sponge |
Pre-operative preparation
Most (peri)pancreatic collections spread to the lesser sac and both paracolic gutters (9,10). Our approach description is based on the left-side approach. Although the right-side approach is very similar, the particularities are later described.
The patient is placed in a right lateral decubitus, the patient body tilted to 40–60° angle, and the left arm flexed with 90° abduction. All pressure sites must be cradled on bean bags. The surgical team is organized as in Figure 2. The percutaneous drain should be cut 2–3 cm over the skin level and the abdomen is prepared in case of emergency laparotomy. A two-percent chlorhexidine and isopropyl alcohol solution is used.
Surgical technique
Retroperitoneal access and trocar position
A 1.5 cm skin incision is made where the percutaneous drain is located. After being cannulated with a 0.035 guidewire, the drain is removed.
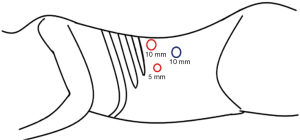
The incision is dilated with the finger until the 10-mm trocar (without the obturator) slides without resistance. Under laparoscopic view, the retroperitoneal insufflation is set up to 10 mmHg. A second 10-mm trocar (surgeon’s right hand) is placed under the 12th costal border over the posterior axillary line. The 5-mm trocar (surgeon’s left hand) is inserted keeping an ergonomic triangulation with the other trocars. Ideally over the anterior axillary line, as shown in Figure 3. Employing the laparoscope and insufflation, the view and identification of structures are enhanced.
Debridement
A fenestrated atraumatic grasper and the irrigation/suction handpiece are simultaneously employed. Debridement of the necrotic tissue should be started from lateral to medial, avoiding the resection of the strongly fixed tissue. The target necrotic tissue has a peculiar dark gray tone. The irrigation of hydrogen peroxide solution highlights the devitalized tissue, contributes to the blunt dissection, and provides hemostasis. Moreover, saline solution is irrigated for clearing the liquid debris. In case of bleeding, packing is usually successful; otherwise, the ultrasonic scalpel (bipolar device is another option) allows hemostatic control with a low risk of injury.
Drains placement and wound closure
A 24–28 Fr silicon drain is inserted under direct vision through a 10-mm trocar and handed with the grasper to be placed in a distal location into the necrotic cavity. An accessory 10 Fr drain could be introduced under direct view through the 5-mm trocar. It can be used for irrigation. The incision of the optical trocar is closed in one layer stitching the lumbodorsal and transversalis fascia using a figure-of-8 suture of a 2-0 slowly absorbable monofilament material. The skin is closed, and the drains fixed to the skin. The dissected tissue and collection sample should be sent for pathology and microbiology examination.
Post-operative management
In cases where a single intervention appears to be enough, we recommend intermittent irrigation through the 10–19 Fr drain with 200–500 mL 0.9% saline three times a day. In those cases with increased residual necrotic tissue, we prefer continuous irrigation with a total of 5–8 liters per day. Our personal indication is to remove the 10–19 Fr drain when the output fluid becomes clear. The 19–28 Fr drain should be removed when the output fluid is less than 30 mL/24 h and a fluid sampling with normal amylase levels. A CT scan is conducted between 1–2 weeks after the procedure. Residual collections should be reassessed and managed according to the step-up approach, based on location, size, and patient conditions (11).
Ethical statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s), and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and accompanying image and video. A copy of the written consent is available for review by the editorial office of this journal.
Tips and tricks
Route planning
In cases with multiple percutaneous drainages, choose the drain with the most direct access to the largest collection cavity as guide for the first trocar.
CO2 insufflation
The retroperitoneal insufflation must be at a maximum pressure of 10 mmHg in order to avoid bacterial translocation.
Dissection
Dissection must be focused on loose necrotic tissue, preserving the vitalized and vascularized pancreatic tissue.
Body and tail pancreas necrosis: the left-side dissection is limited by the posterior gastric wall anteriorly, the inferior pole of the spleen, and the anterior renal fascia posteriorly, having the transverse colon with its mesocolon as the inferior limit (Figure 4). Take care of the splenic artery and its branches crossing this plane to the splenic hilum.
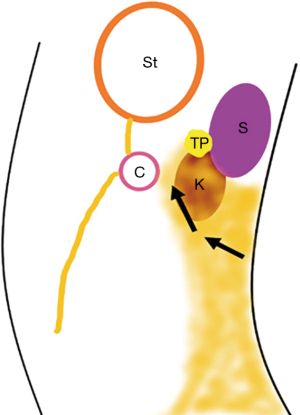
Head and neck pancreas necrosis: the right-side dissection is limited by the parietal peritoneum and the duodenum anteriorly, and the anterior renal fascia as the posterior limit. The access to the head of the pancreas requires careful dissection of the retroperitoneum, creating a retroduodenal window. Due to the technical difficulty, these cases are usually more suitable for the endoscopic approach.
Paracolic gutter collections: the inferior left/right side dissection is limited anteriorly by the parietocolic ligament, the ascending/descending colon, and the mesocolon. As posterior limit the anterior renal fascia (Figure 5). Running through this path, the collection spreads to the pelvic cavity.
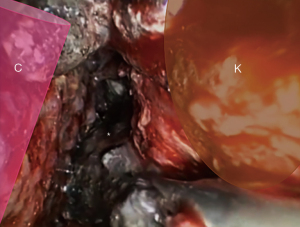
Perirenal abscess: it could be present a secondary perirenal abscess by the dissemination/translocation of the collection to the perirenal space. The anterior renal fascia must be incised for the aspiration of the collection and, if deemed necessary, drain placement (Figure 6).
Bleeding
Most of the dissection’s bleeding corresponds to capillary ruptures. The first measures are irrigation with hydrogen peroxide solution, transitory packing, and the use of energy devices. Only if these actions do not seem to be enough, an absorbable hemostatic sponge may be placed. Caution should be taken not to place the hemostatic sponge too close to the drains, to avoid clogging (see the Video 1).
The principal advantages and complications of this approach are listed below (Tables 3,4).
Table 3
| Reduce the number of interventions |
| Hemostatic control |
| Instruments familiar to surgeons |
| Avoids peritoneal cavity contamination |
| Minimal traumatic incisions |
Table 4
| Bleeding |
| Pancreatocutaneous fistula |
| Colonic fistula |
| Colon necrosis |
Conclusions
Currently, there are multiple options into the step-up approach for infected pancreatic necrosis, hence the accurate selection of the patient candidate for laparoscopic retroperitoneal necrosectomy is of most importance. Although this technique requires mastery of retroperitoneal anatomy and good laparoscopic skills, it is an exceptional tool for the surgeon within the arsenal for this difficult and serious condition. An additional advantage is the feasibility at any time without requiring radiologic or endoscopic intervention. Employing this laparoscopic technique, the view and identification of the anatomical structures are enhanced, as compared to other video-assisted one port approaches. The authors have adopted the laparoscopic retroperitoneal necrosectomy as part of the step-up approach and have found it very replicable and ergonomic.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://asj.amegroups.com/article/view/10.21037/asj-21-101/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-21-101/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s), and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and accompanying image and video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leppäniemi A, Tolonen M, Tarasconi A, et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg 2019;14:27. [Crossref] [PubMed]
- Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Werge M, Novovic S, Schmidt PN, et al. Infection increases mortality in necrotizing pancreatitis: A systematic review and meta-analysis. Pancreatology 2016;16:698-707. [Crossref] [PubMed]
- De Waele JJ. A step-up approach, or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010;363:1286-7. [Crossref] [PubMed]
- Logue JA, Carter CR. Minimally Invasive Necrosectomy Techniques in Severe Acute Pancreatitis: Role of Percutaneous Necrosectomy and Video-Assisted Retroperitoneal Debridement. Gastroenterol Res Pract 2015;2015:693040. [Crossref] [PubMed]
- Sion MK, Davis KA. Step-up approach for the management of pancreatic necrosis: a review of the literature. Trauma Surg Acute Care Open 2019;4:e000308. [Crossref] [PubMed]
- Fong ZV, Fagenholz PJ. Minimally Invasive Debridement for Infected Pancreatic Necrosis. J Gastrointest Surg 2019;23:185-91. [Crossref] [PubMed]
- Rasslan R, Novo FDCF, Bitran A, et al. Management of infected pancreatic necrosis: state of the art. Rev Col Bras Cir 2017;44:521-9. [Crossref] [PubMed]
- Zhao G, Hu M, Liu R, et al. Retroperitoneoscopic Anatomical Necrosectomy: A Modified Single-Stage Video-Assisted Retroperitoneal Approach for Treatment of Infected Necrotizing Pancreatitis. Surg Innov 2015;22:360-5.
- Ulagendra Perumal S, Pillai SA, Perumal S, et al. Outcome of video-assisted translumbar retroperitoneal necrosectomy and closed lavage for severe necrotizing pancreatitis. ANZ J Surg 2014;84:270-4. [Crossref] [PubMed]
- Liu ZW, Yang SZ, Wang PF, et al. Minimal-access retroperitoneal pancreatic necrosectomy for infected necrotizing pancreatitis: a multicentre study of a step-up approach. Br J Surg 2020;107:1344-53.
Cite this article as: Marmolejo A, Romero JA, Farell J, Ruiz-Funes AP. Minimally invasive retroperitoneal necrosectomy: how do we do it? AME Surg J 2023;3:8.




