
Management of radiation-induced angiosarcoma of the breast with total mastectomy and superficial inferior epigastric artery flap reconstruction: a case report
Introduction
Radiation-induced angiosarcoma (RIAS) of the breast is a rare, long-term complication of radiation therapy. It is, however, a clinically significant disease with a high recurrence rate and poor prognosis. It has been reported to occur in 0.01–0.4% of patients treated with adjuvant radiotherapy for a previous breast cancer (1,2). Given its rarity, much of the available literature on RIAS is described in case reports and case series. Many articles, however, note a concern for increased incidence in RIAS due to increased use of adjuvant radiation after breast conservation surgery over the last three decades (2-6). Many patients have received previous reconstructive procedures for their initial breast cancer and thus the surgical management and reconstructive options must be considered when discussing a treatment plan with patients.
We present a case of a 54-year-old woman who received a total mastectomy for RIAS with subsequent breast reconstruction with superficial inferior epigastric artery (SIEA) flap in accordance with the CARE reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-21-103/rc).
Case presentation
A 54-year-old female presented to our clinic with complaints of a persistent and expanding bruise on her left breast. The patient has a pertinent history of asthma and more than a 10-pack year history of tobacco use. Her family history is significant for a maternal grandmother with breast cancer diagnosed in her 70 s and a paternal half-sister with fibrolamellar hepatocellular carcinoma diagnosed at age 17.
Previously, at age 47, the patient had presented for evaluation of a palpable lump on her left breast. Bilateral mammogram and left breast ultrasound demonstrated a 4 cm irregular mass at the 3:00 position, as well as an enlarged axillary node measuring 3 cm. She underwent ultrasound-guided breast and axillary node biopsies which revealed a poorly differentiated invasive ductal carcinoma, estrogen receptor (ER) and progesterone receptor (PR) positive, human epidermal growth factor receptor (HER2/neu)-negative and positive axillary lymph node for metastatic carcinoma. Staging with computer tomography (CT) and bone scan demonstrated no evidence of distant metastatic disease. She completed neoadjuvant chemotherapy with Doxorubicin and Cyclophosphamide followed by Paclitaxel (ddAC-ddT). She ultimately underwent left lumpectomy and axillary dissection with concurrent bilateral oncoplastic reduction. Pathology confirmed her diagnosis and revealed negative margins, however, two of seventeen lymph nodes were positive for metastatic carcinoma. She went on to complete adjuvant radiotherapy and began Tamoxifen. Her radiation treatment was to the left breast with tangential fields using field-in-field technique. She received 180 cGy per day to 4,500 cGy in 25 fractions over the span of one month.
At age 52, the patient was found to have another mass in the upper inner quadrant of her right breast. Ultrasound-guided biopsy revealed poorly-differentiated, triple-negative, invasive ductal carcinoma. She underwent lumpectomy and sentinel node biopsy with concurrent oncoplastic reduction of the right breast. Pathology of right breast lumpectomy revealed a 0.45 cm poorly differentiated invasive ductal carcinoma, clear margins, and negative nodes. She later declined adjuvant therapy and was ultimately lost to follow-up.
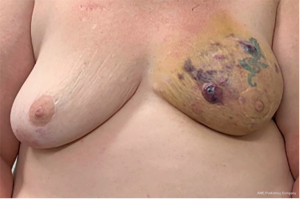
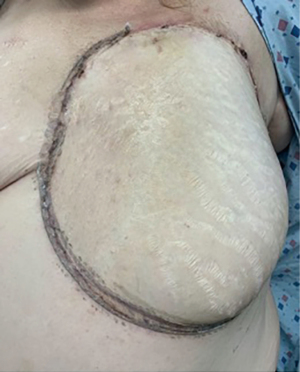
Two years later, the patient presented to our clinic with a four-month history of bruising of the left breast, progressive skin changes, and pain (Figure 1). She had undergone a punch biopsy at an outside clinic which had revealed high grade angiosarcoma. Her workup included staging CT and positron emission tomography (PET) which did not reveal evidence of distant metastasis. The patient underwent a left total mastectomy with immediate, autologous reconstruction with SIEA flap (Figure 2). Her pathology was consistent with 15 cm × 14 cm, high grade angiosarcoma of the breast with involvement of the skin and subareolar tissue, and 4 cm extension into the underlying breast parenchyma. Surgical margins were negative measuring ≥3 cm on pathology, with no evidence of disease in surrounding skeletal muscle. Unfortunately, the patient presented with five abdominal cutaneous lesions 7 months after initial diagnosis, which was subsequently confirmed to be recurrence of her angiosarcoma on punch biopsy.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
RIAS of the breast is a rare and aggressive complication of radiation therapy. To our knowledge, no studies have assessed specific radiation doses that increase the risk of angiosarcoma development, owing to the rare nature of the cancer and lack of prospective studies. However, several cases of RIAS have been reported to develop in tissue surrounding the direct radiation field, hypothesizing that these peripheral areas receive radiation levels below the threshold for apoptosis but enough to induce genetic instability leading to development of RIAS (4,7). The most common reported radiation dose among these studies is 50 Gy (4,6,8,9), though this data is missing from a majority of publications (2).
The average latency between adjuvant radiotherapy and development of cutaneous RIAS symptoms is 6 years (2,3,10), though is reported to arise anywhere from 3 to 19 years after radiation (1,11). The median age at diagnosis is 67–71 years of age (range, 36–92 years) (3,4,8,12,13). Our patient developed RIAS 5.5 years after completion of radiotherapy. RIAS typically appears as purple-pink or purple-blue ecchymosis or petechial cutaneous lesions, which can range from 1 to 15 cm in size. These lesions are often misattributed to local trauma or other benign breast conditions; diagnosis is typically considered once the lesion does not resolve or grows in size. Physical examination did not reveal a palpable breast mass or lymphadenopathy in our patient, which is also consistent with the literature.
Many of the typical imaging modalities used for screening and diagnosis are not sensitive for RIAS lesions. Both mammography and ultrasound inconsistently show defined lesions, often because other radiation-related tissue changes lend to poor visualization of a subcutaneous lesion (3,14). Magnetic resonance imaging (MRI) is likely more sensitive; however, it is not part of routine screening for women with a history of breast cancer, unilateral mastectomy and/or breast reconstruction per American College of Radiology’s new 2020 guidelines (15,16). MRI can be used for surgical planning and disease staging (17). Fine-needle aspiration also has been shown to produce false-negatives (6,14). Diagnosis is most commonly confirmed with histopathologic evaluation of a core-needle or punch biopsy. However, a low threshold of suspicion must be present to consider this diagnostic test, especially given many of the initial findings of an aggressive disease are not typically present in these sarcomas. The rare nature of RIAS, the heterogeneity of presenting symptoms, and the long latency period between initial breast cancer therapy and appearance of RIAS often delays tissue biopsy and clinical diagnosis (5). Our patient received a confirmatory biopsy two months after the appearance of her symptoms. Unfortunately, due to issues regarding insurance coverage, surgical treatment was delayed another three months.
Current management of RIAS in the literature involves largely surgical excision, with a minority of studies reporting adjuvant radiation or chemotherapy regimens (3,4,8,17). Much of the literature demonstrates that negative margins are not sufficient to prevent local recurrence (13). British Sarcoma Group guidelines for surgical management of angiosarcoma emphasizes aggressive excision with widest possible margins (18), which may include the underlying prepectoral fascia and muscle if needed. Lindford et al. suggested macroscopic margins greater than 3 cm and deep margins extending to prepectoral fascia (5,19,20). Feinberg et al. reported patients in specialized sarcoma centers were more likely to receive a more thorough excision of irradiated tissue, which was correlated with increased overall survival (10). Rate of local recurrence was also found to be lower in patients with increasing margin width, leading the authors to suggest that excision of irradiated tissue is more important than adequate surgical margins (10).
Some studies note a survival benefit with adjuvant radiotherapy, however, this option remains controversial (3,10), owing to a potential selective publication bias (2). Additionally, given the rare nature of the disease, various adjuvant chemotherapy regimens have been postulated, but with limited evidence of survival benefit given the lack of prospective, randomized trials (3,12). There are few cases reporting management of RIAS with radiotherapy or chemotherapy alone, often because the patient’s disease burden or comorbidities rendered them unfit for surgery. Not surprisingly, recurrence and mortality in this group of patients are high (2).
The major concern for most surgeons in the short term is persistently positive margins necessitating multiple reoperations. Our patient’s high BMI and adequate abdominal adiposity enabled for aggressive wide local excision and autologous free tissue transfer to help ensure negative margins and provide ideal wound coverage. She was managed with total mastectomy and margins were confirmed to be 3 cm on histopathology.
At the time of surgical consultation for breast reconstruction for our patient, a deep inferior epigastric perforator (DIEP) flap was discussed. However, the decision was made intraoperatively to reconstruct the left breast with a SIEA flap instead. The SIEA flap is an innovative autologous reconstruction technique that involves the superficial inferior epigastric vessels and surrounding abdominal tissue anterior to the fascial layers of the abdomen. As the anatomic name suggests, these vessels supply the abdominal tissue from a more superficial pedicle, whereas branching vessels of the deep inferior epigastrics perforate through the abdominal fascia from a deeper layer. The concern with DIEP flap is that once it is transferred and supplying vessels are anastomosed, the weight of the tissue would occlude the main vessels or smaller caliber perforators, leading to complications such as pedicle thrombosis or flap necrosis. Large SIEA flaps are theoretically less likely to compress their own blood supply under the weight of the flap after tissue transfer because of their more superficial location within the tissue. In our patient, the resection of the sarcoma as part of her total mastectomy left a large surface area to be covered, and upon flap harvest, the weight of the tissue needed for adequate coverage exceeded 2 kilograms. Given the patient’s large body habitus, the abdominal wall complications cited with DIEP flap harvest, and presence of adequately sized superficial artery with a palpable pulse, the plastic surgeon determined the most appropriate alternative to the planned DIEP flap would be the SIEA flap in this patient.
The necessity for extensive surgical resection for RIAS, typically also after surgical resection of a primary breast cancer, lends to sparse reconstructive options for women affected by this often extensive, secondary cancer. To date, there have been limited studies discussing breast reconstruction after surgical resection of RIAS, those that do mention local pedicled flaps or immediate versus delayed free flap options (4,5,14). Hanasono et al. focused on recurrence of the disease after autologous tissue transfer and recommended delayed reconstruction to allow for proper follow up and monitoring for disease recurrence (14). However, the author’s case example utilized wide local excision and split thickness skin graft (STSG) for coverage and did not suggest other methods of coverage of larger defects left by a more radical resection (14). Recurrence within the autologous tissue has only been reported on rare occasions (14). Recommendations on appropriate options and timing of reconstruction are difficult to elucidate, even considering patient factors that may increase the odds of reoperation should local recurrence occur.
The argument for immediate reconstruction supports the use of autologous tissue, namely that it offers the most coverage after resection of irradiated tissue. Additional consideration must be given, however, if the patient had received autologous reconstruction during her previous cancer treatment (9). This was not the case in our patient who had received oncoplastic reduction at the time of her previous breast surgeries. Other options include local flap coverage or skin grafting. However, Jallali et al. cautioned against STSG as the primary method for reconstruction and defect coverage after RIAS excision, citing concerns for graft take in previously-irradiated tissue (4). Authors additionally advocated for use of flaps given their low complication rate and donor site morbidity in their retrospective review of 12 patients (4). Crosby et al. also supported the use of an abdominal or gluteal free flap (or latissimus dorsi flap with implant) for immediate reconstruction after total mastectomy and history of radiation (1).
Despite treatment efforts, RIAS confers a poor prognosis, and survival rates are reported anywhere between 10% and 43% after 5 years with a median of 23–37 months survival for patients (2,14,19). Local recurrence has been reported to occur in 45–64% of patients, while distant metastasis rates occur in 27–42% of cases with high mortality (3,13,19). The average time to local recurrence is 6 months, and recurrence-free survival is reported as 18 months (13,19). Prognostic factors include tumor size, age, grade, and presence of local recurrence (2,3,14). Early diagnosis and prompt removal also may decrease mortality (8), however, the high rate of recurrence of this cancer necessitates frequent monitoring for signs of local recurrence. The most common sites of metastasis are the lung and liver (12,20). Many institutions have described their post-operative management as including either CT or MRI to locate any distant metastases or disease dissemination.
The emotional toll of RIAS on a patient can be quite severe. The patient in our case developed angiosarcoma as a third cancer of her breasts within 10 years. The patient cited emotional exhaustion on her clinical follow-up, particularly after appearance of additional lesions. RIAS is a rare complication and its management needs to be patient-tailored and well planned. Though limited by rare reporting and lack of prospective clinical trials, much of the outcomes in the literature support radical resection of breast tissue as it is correlated with reduced rates of local recurrence and improved survival (9,10). Consultation to plastic and reconstructive surgery should occur, as wide excision of irradiated tissue requires complex coverage, preferably with an autologous flap. This case is an example of a planned multi-disciplinary management approach with breast surgical oncology, plastic surgery, medical oncology, and radiation oncology to best serve the patient.
RIAS of the breast is a highly morbid outcome of adjuvant radiation for breast cancer. Though rare, the rapidly progressive and recurrent nature of this disease necessitates that clinicians caring for patients with a history of radiation breast cancer consider RIAS as a possibility when a patient presents with a persistent, seemingly benign skin lesion near the radiated field. Once diagnosed, surgical treatment should not be delayed. Management option of RIAS of the breast is limited, but should include wide-local excision or mastectomy. We have described a case managed with total mastectomy and SIEA flap with success to add to the medical literature. Ultimately, surgery should focus on wide excision of previously-radiated tissue with potential for autologous tissue transfer. Patients should be followed closely for local recurrence and distant metastasis. A patient-centered approach is necessary for treatment compliance and to reduce the emotional burden of this potentially aggressive, secondary disease.
Acknowledgments
We would like to acknowledge Richard Youn, MD for his assistance in the research and clinical expertise on which the work was based.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-21-103/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-21-103/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Crosby MA, Chike-Obi CJ, Baumann DP, et al. Reconstructive outcomes in patients with sarcoma of the breast. Plast Reconstr Surg 2010;126:1805-14. [Crossref] [PubMed]
- Depla AL, Scharloo-Karels CH, de Jong MAA, et al. Treatment and prognostic factors of radiation-associated angiosarcoma (RAAS) after primary breast cancer: a systematic review. Eur J Cancer 2014;50:1779-88. [Crossref] [PubMed]
- Chesebro AL, Chikarmane SA, Gombos EC, et al. Radiation-Associated Angiosarcoma of the Breast: What the Radiologist Needs to Know. AJR Am J Roentgenol 2016;207:217-25. [Crossref] [PubMed]
- Jallali N, James S, Searle A, et al. Surgical management of radiation-induced angiosarcoma after breast conservation therapy. Am J Surg 2012;203:156-61. [Crossref] [PubMed]
- Lindford A, Böhling T, Vaalavirta L, et al. Surgical management of radiation-associated cutaneous breast angiosarcoma. J Plast Reconstr Aesthet Surg 2011;64:1036-42. [Crossref] [PubMed]
- Virgilio E, Lombardi M, Stefano DD, et al. Angiosarcoma of the Breast: A Rare and Dismal Complication of Breast Surgery Associated with Radiation. Am Surg 2017;83:e71-73. [Crossref] [PubMed]
- Neuhaus SJ, Pinnock N, Giblin V, et al. Treatment and outcome of radiation-induced soft-tissue sarcomas at a specialist institution. Eur J Surg Oncol 2009;35:654-9. [Crossref] [PubMed]
- Hanna G, Lin SJ, Wertheimer MD, et al. Unresolved, atraumatic breast hematoma: post-irradiation or secondary breast angiosarcoma. Breast Dis 2011;33:139-42. [Crossref] [PubMed]
- Yip C, Weiler-Mithoff E, Doughty JC, et al. Radiation-associated angiosarcoma after autologous breast reconstruction: report of two cases in a plastic surgery unit. Eur J Plast Surg 2019;42:513-6.
- Feinberg L, Srinivasan A, Singh JK, et al. Impact of specialist management on survival from radiation-associated angiosarcoma of the breast. Br J Surg 2018;105:401-9. [Crossref] [PubMed]
- Eppelheimer CN, Marti JL, Eisenberg A, et al. A Case of Secondary Angiosarcoma of the Breast after Breast-conserving Surgery and Radiation: Review of Radiologic and Pathologic Findings. J Clin Imaging Sci 2015;5:45.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol 2013;20:1267-74.
- Abbott R, Palmieri C. Angiosarcoma of the breast following surgery and radiotherapy for breast cancer. Nat Clin Pract Oncol 2008;5:727-36.
- Hanasono MM, Osborne MP, Dielubanza EJ, et al. Radiation-induced angiosarcoma after mastectomy and TRAM flap breast reconstruction. Ann Plast Surg 2005;54:211-4. [Crossref] [PubMed]
- American College of Radiology. ACR Appropriate Criteria imaging after mastectomy and breast reconstruction. Accessed: September 7, 2021. Available online: https/acsearch.acr.org/docs/3155410/Narrative
- Early AP, Moon W. Breast Cancer and Secondary Cancer Recurrences After Autologous Tissue Reconstruction. Clin Breast Cancer 2021;21:e96-e101. [Crossref] [PubMed]
- Wei NJJ, Crowley TP, Ragbir M. Early Breast Angiosarcoma Development After Radiotherapy: A Cautionary Tale. Ann Plast Surg 2019;83:152-3. [Crossref] [PubMed]
- Grimer R, Judson I, Peake D, et al. Guidelines for the management of soft tissue sarcomas. Sarcoma 2010;2010:506182. [Crossref] [PubMed]
- Seinen JM, Styring E, Verstappen V, et al. Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol 2012;19:2700-6. [Crossref] [PubMed]
- Morgan EA, Kozono DE, Wang Q, et al. Cutaneous radiation-associated angiosarcoma of the breast: poor prognosis in a rare secondary malignancy. Ann Surg Oncol 2012;19:3801-8. [Crossref] [PubMed]
Cite this article as: Haffner Z, Sogunro O, Tansey J, Greenwalt I, Song DH. Management of radiation-induced angiosarcoma of the breast with total mastectomy and superficial inferior epigastric artery flap reconstruction: a case report. AME Surg J 2023;3:9.


