
Primary angiosarcoma of the breast: a case report
Introduction
Angiosarcoma of the breast is an extremely rare neoplasm, accounting for less than 0.05% of all breast malignancies (1). Based on etiology, it can be divided into primary and secondary breast angiosarcoma. Primary breast angiosarcoma occurs sporadically primarily in young women and often presents as a palpable mass. Secondary breast angiosarcoma most frequently develops in older patients with prior radiation therapy or chronic lymphedema. This aggressive malignant tumor of the vascular endothelium is characterized by rapid proliferation and infiltrating growth patterns (2). Given its high risk of local recurrence and distant metastasis, the tumor portends a poor prognosis (3). Complete surgical resection of the tumor is the mainstay of treatment as there is a lack of consensus regarding systemic therapies (4). Here, we present a case of primary breast angiosarcoma that initially masqueraded as a benign vascular lesion on core needle biopsy, was ultimately diagnosed on excisional biopsy performed after the mass began to rapidly enlarge and was definitively managed with mastectomy. We present the following case in accordance with the CARE reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-21-111/rc).
Case presentation
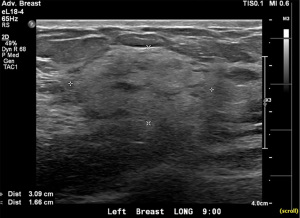
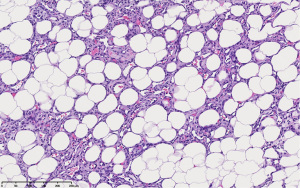
A 46-year-old female presented after palpating a mass of the medial left breast. She had no other breast symptoms and no personal history of breast surgery or irradiation. Family history was negative for breast, ovarian, and pancreatic cancers but positive for colon cancer in her grandmother. On examination, a 3–4 cm round rubbery mobile mass was palpated in the upper inner quadrant of the left breast. The mass was nontender and there was no evidence of skin changes, nipple retraction or axillary lymphadenopathy. Mammogram demonstrated a new 4.2 cm ill-defined region of increased density corresponding to the palpable abnormality (BI-RADS 4). Targeted ultrasound in this region demonstrated a 3.9 cm × 3.1 cm × 1.7 cm lesion for which biopsy was recommended (Figure 1). Ultrasound-guided biopsy was performed and pathology showed vascular channels lined by cytologically bland appearing endothelial cells rendering diagnosis of a benign vascular lesion with main considerations including capillary hemangioma and angiolipoma (Figure 2). On immunohistochemistry analysis, the lesion was positive for CD31, CD34 and factor VIII.
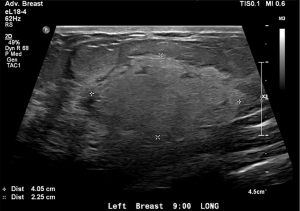
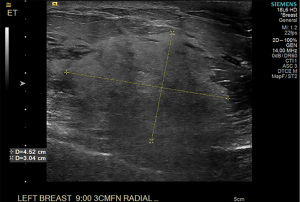
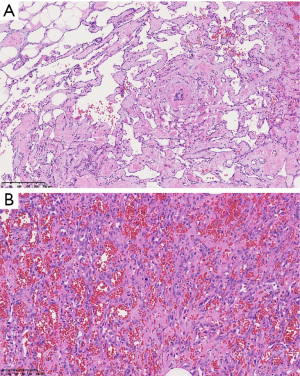
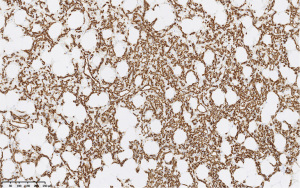
The patient was seen for initial surgical consultation after being referred by her radiologist. However, she deferred surgery considering the COVID-19 pandemic. Her 6-month follow-up ultrasound of left breast demonstrated that the lesion increased in size to 4.3 cm × 4.1 cm × 2.3 cm (Figure 3). Repeat imaging at 1 year showed interval growth of vascular lesion to 4.5 cm × 4.1 cm × 3 cm (Figure 4). Given progressive growth, surgical excision was again recommended and the patient underwent left breast excisional biopsy. Pathology showed angiosarcoma that was 4.1 cm in size. On microscopic examination, the tumor morphologically appeared moderately differentiated with prominent anastomosing channels, papillary growth, endothelial tufting, cytologic atypia, focal solid areas and focal blood lakes (Figure 5A,5B). Immunohistochemistry was positive for CD31, CD34 and factor VIII (Figure 6). Multiple margins were noted to be positive.
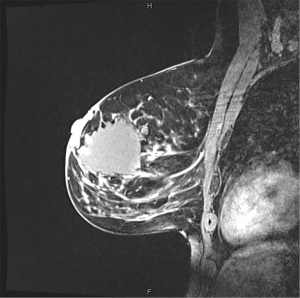
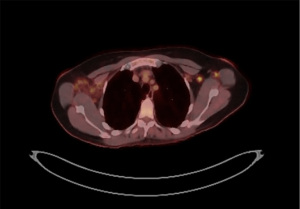
The patient was presented at multi-disciplinary sarcoma tumor board. Recommendations were made for further staging imaging. Post-surgical breast MRI showed thick nodular enhancement surrounding surgical cavity in left breast suspicious for residual disease with and multiple prominent left axillary lymph nodes coinciding with hypermetabolic activity on restaging positron emission tomography (PET) scan (Figures 7,8). CT showed morphologically normal but fluorodeoxyglucose (FDG) avid lymph nodes in left axilla. No radiographic evidence of distant metastases was found. Bilateral second-look ultrasound showed a lateral right breast sub-centimeter mass with adjacent punctate calcifications that was biopsied revealing benign breast tissue. Pathology and imaging were concordant. A right breast diagnostic mammogram and ultrasound were obtained to ensure adequate sampling of microcalcifications and showed two groupings of calcifications at 8 o’clock posterior depth and a 1 cm hypervascular mass adjacent to the biopsy marking clip for which excision was recommended.
Given these findings, surgery was recommended to achieve negative margins and excise the newly found right breast 1 cm hypervascular mass. She was taken to the operating room and underwent left skin sparing mastectomy with left axillary sentinel lymph node biopsy given PET findings of avid axillary lymph nodes and right breast excisional biopsy followed by left breast DIEP flap reconstruction and right breast oncoplastic reduction. Pathology of the left breast revealed 2 cm residual angiosarcoma adjacent to prior lumpectomy cavity with a 1.5 mm span of angiosarcoma present at the anterior margin and lymph nodes were negative for metastasis. Right breast pathology was benign, showing no evidence of atypical proliferation or malignancy. She subsequently underwent successful wide local excision of left breast positive skin margin to attain clear margins and reduce local recurrence risk. Patient did well post-operatively and final pathology revealed no residual disease. Her follow up plan includes clinical examinations every 3 months, imaging surveillance with CT chest every 3 months for 2 years, then every 6 months for 5 years, and then annually, routine screening mammography of the right breast, and no further adjuvant therapy.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Angiosarcoma of the breast is a malignant vascular neoplasm originating from endothelium lining vascular channels that occurs as clinically distinct primary and secondary forms. Primary breast angiosarcoma is very rare and accounts for 0.05% of all malignant breast cancers (5). The tumor typically arises in the breast parenchyma of young women between 30 and 50 years of age with no history of radiation or any other known risk factors (1). Secondary breast angiosarcoma arises in the dermal and subcutaneous layers of the skin after a period of 5 to 10 years following radiation therapy and in association with chronic lymphedema resulting from axillary dissection (6). The annual incidence of primary breast angiosarcoma is approximately 17 new cases per million women and the diagnosis is generally associated with a poor prognosis (7).
Clinically, breast angiosarcoma often presents insidiously as a painless, palpable mass with rapid growth. Bluish red discoloration of overlying skin is seen in up to a third of patients and is thought to be a result of the vascular nature of the tumor (6). Nipple discharge or retraction and axillary lymphadenopathy are usually absent. In most reported cases, the average tumor size at presentation is greater than 4 cm in diameter. Bilateral tumors have been reported in postmenopausal women (1). Differential diagnoses range from benign breast conditions such as hemangioma, angiolipoma, and pseudoangiomatous stromal hyperplasia (PASH) to malignancies such as pseudovascular metaplastic breast carcinoma and acantholytic variant squamous cell carcinoma (8).
Imaging of angiosarcoma is relatively non-specific (9). On mammography, angiosarcoma appears as an ill-defined, non-calcified mass with a mean size of 4.5 cm (1). Sonography shows a solid mass with well-defined or lobulated margins (1). Magnetic resonance imaging (MRI) shows a mass with low intensity signal on T1 weighted images and high intensity signal on T2 weighted images suggesting presence of blood flow in vascular channels (1). This study is useful for determining the extent of tumor, surgical planning, and, in the case of our patient, detecting residual disease (6). Retrospectively, addition of a breast MRI during initial work-up of our patient may have aided in earlier characterization and diagnosis of the lesion. PET with 18F-FDG or CT chest is useful for staging of angiosarcoma with the most common site of metastasis being pulmonary metastases (6).
Angiosarcoma may be misinterpreted as a benign lesion particularly in younger patients secondary to its rarity and often deceptively benign histologic appearance (9,10). In this case, initial ultrasound-guided core needle biopsy was interpreted as a benign vascular lesion with main considerations including capillary hemangioma and angiolipoma. On histology, benign vascular lesions such as hemangiomas are comprised of well-formed vascular channels without dissecting growth patterns (5). Cytologic features of angiosarcoma are wide ranging and include anastomosing vascular channels with infiltrative architecture, endothelial multilayering, extravasation of blood from malignant vessels forming blood lakes, nuclear atypia, abnormal mitoses, and necrosis (11). Immunostaining for factor VIII and CD31 endothelial markers indicates vascular differentiation but is not specific to malignant vascular lesions (9). Ki-67 proliferation index and immunoreactivity for Skp2 have been described as diagnostic tools to distinguish between benign and malignant vascular lesions of the breast (12). Hypothetically, a lower Ki-67 proliferation index and absence of Skp2 expression may be helpful in differentiating hemangiomas from angiosarcomas on a case-by-case basis (12). This case demonstrates the importance of having a high index of suspicion for angiosarcoma particularly in the setting of infiltration of vascular channels through adipose tissue on biopsy.
Pre-operative diagnosis of angiosarcoma by fine needle aspiration or core needle biopsy may be difficult due to insufficient sampling of the tumor. In one review, the malignant nature of the tumor was not recognized in more than a third of initial percutaneous biopsy specimens (10). These tumors were misdiagnosed as benign lesions such as hemangioma, lymphangioma, hematoma or dysplasia (10). In some cases, the delay in correct diagnosis was up to two years (10). Macrobiopsy provides a larger sample but can be challenging to perform secondary to the highly vascular nature of the tumor. Surgical resection is frequently necessary for adequate sampling to establish a final diagnosis. Pathologists play a key role in the diagnosis of angiosarcoma, which is confirmed by histological examination. Accurate histopathological diagnosis is critical and studies support obtaining further expert opinion from a specialist soft tissue sarcoma pathologist in select cases (13).
Given the rarity of angiosarcoma of the breast, standardized treatment has not yet been established. As primary angiosarcoma has a high rate of local recurrence, current treatment strategy is complete excision of the tumor (14). Surgical resection with mastectomy is the mainstay of treatment for both primary and secondary forms (1). Radical surgery is favored for primary angiosarcoma with an estimated risk of recurrence of 8% as opposed to 23% after breast conserving treatment (15). Similarly, radical resection of radiation-associated angiosarcoma of the breast is associated with reduced recurrence rates and improved disease-specific survival (16). Primary breast angiosarcoma primarily metastasizes via the hematogenous route to lungs, liver, bones, skin and the contralateral breast (1). However, lymph node metastasis has been reported and lymphadenectomy or axillary dissection is performed with mastectomy if suspected (14).
The COVID-19 pandemic poses many clinical challenges including abatement of cancer screening and delay in oncological treatment. Our patient initially refused surgery secondary to fear of COVID-19 contagion. Anxiety related to the COVID-19 outbreak has been shown to impair proper clinical management with higher rates of surgical refusal among patients with suspicious breast lesions or even breast cancer (17). In a large retrospective study, refusal of cancer directed surgery by breast cancer patients was associated with more than double the risk of mortality (18). Providers should notify patients of the risks associated with surgical refusal and treatment delay and the potentially detrimental effects on long-term clinical outcomes.
Given poor overall outcomes and high risk of metastasis in patients with primary angiosarcoma of the breast, multimodal therapy with surgery, systemic chemotherapy, and radiation therapy has been suggested in recent literature. Chemotherapy may reduce the rate of local recurrence and increase rates of complete pathologic response (6,19). A systematic review of literature on angiosarcoma showed a beneficial role of neoadjuvant chemotherapy on downsizing tumors resulting in improved resection margins but showed no clear survival benefit (20). Radiation therapy may improve regional control and survival (7). Given the high incidence of local recurrence, adjuvant radiation therapy may be considered as a possible therapeutic option. Kronenfeld et al. published an analysis of clinical outcomes of primary angiosarcomas and radiation-associated angiosarcomas of the breast treated with neoadjuvant chemotherapy, radiation in select patients, surgery, and adjuvant chemotherapy (19). They concluded that this multimodal treatment approach may lead to improved outcomes including prolonged survival (19). Further investigation of the role of systemic therapy with prospective, multi-institutional studies is warranted to overcome the limitations associated with the low incidence of the disease (21).
Conclusions
Primary angiosarcoma of the breast is a rare but aggressive disease that develops in patients without prior history of breast irradiation or other risk factors with a high chance of local recurrence and metastasis. A large, highly vascular breast mass should be considered malignant until proven otherwise. Ultrasound-guided core needle biopsy and excisional biopsy of larger lesions aids in obtaining adequate sampling for an accurate diagnosis. The most effective treatment modality remains complete surgical resection with negative margins. In many cases, mastectomy is required to achieve this endpoint. Breast conservation surgery portends a higher risk of recurrence and the data supporting this approach is limited (15). The role of adjuvant chemotherapy or radiation therapy in the management of angiosarcoma remains to be proven but may be considered in high-risk patients. While randomized controlled trials are needed to improve our understanding of the role of adjuvant treatment options in breast angiosarcoma, the rarity of these lesions makes these trials difficult to accrue and perform.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-21-111/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-21-111/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bhosale SJ, Kshirsagar AY, Patil MV, et al. Primary angiosarcoma of breast: A case report. Int J Surg Case Rep 2013;4:362-4. [Crossref] [PubMed]
- Yin M, Wang W, Drabick JJ, et al. Prognosis and treatment of non-metastatic primary and secondary breast angiosarcoma: a comparative study. BMC Cancer 2017;17:295. [Crossref] [PubMed]
- Masai K, Kinoshita T, Jimbo K, et al. Clinicopathological features of breast angiosarcoma. Breast Cancer 2016;23:718-23. [Crossref] [PubMed]
- Pasta V, Monti M, Cialini M, et al. Primitive sarcoma of the breast: new insight on the proper surgical management. J Exp Clin Cancer Res 2015;34:72. [Crossref] [PubMed]
- Glazebrook KN, Magut MJ, Reynolds C. Angiosarcoma of the breast. AJR Am J Roentgenol 2008;190:533-8. [Crossref] [PubMed]
- Varghese B, Deshpande P, Dixit S, et al. Primary Angiosarcoma Of the Breast: A Case Report. J Radiol Case Rep 2019;13:15-25. [Crossref] [PubMed]
- Desbiens C, Hogue JC, Lévesque Y. Primary breast angiosarcoma: avoiding a common trap. Case Rep Oncol Med 2011;2011:517047. [Crossref] [PubMed]
- Nascimento AF, Raut CP, Fletcher CD. Primary angiosarcoma of the breast: clinicopathologic analysis of 49 cases, suggesting that grade is not prognostic. Am J Surg Pathol 2008;32:1896-904. [Crossref] [PubMed]
- Bordoni D, Bolletta E, Falco G, et al. Primary angiosarcoma of the breast. Int J Surg Case Rep 2016;20S:12-5. [Crossref] [PubMed]
- Chen KT, Kirkegaard DD, Bocian JJ. Angiosarcoma of the breast. Cancer 1980;46:368-71. [Crossref] [PubMed]
- Zhang H, Turner BM, Katerji H, et al. Vascular lesions of the breast: Essential pathologic features and diagnostic pitfalls. Human Pathology Reports 2021;26:300570.
- Shin SJ, Lesser M, Rosen PP. Hemangiomas and angiosarcomas of the breast: diagnostic utility of cell cycle markers with emphasis on Ki-67. Arch Pathol Lab Med 2007;131:538-44. [Crossref] [PubMed]
- Rupani A, Hallin M, Jones RL, et al. Diagnostic Differences in Expert Second-Opinion Consultation Cases at a Tertiary Sarcoma Center. Sarcoma 2020;2020:9810170. [Crossref] [PubMed]
- Sasahara A, Tanabe M, Hayashi K, et al. A case of primary breast angiosarcoma with multiple discontinuous small lesions. Surg Case Rep 2019;5:157. [Crossref] [PubMed]
- Johnson CM, Garguilo GA. Angiosarcoma of the breast: A case report and literature review. Curr Surg 2002;59:490-4. [Crossref] [PubMed]
- Li GZ, Fairweather M, Wang J, et al. Cutaneous Radiation-associated Breast Angiosarcoma: Radicality of Surgery Impacts Survival. Ann Surg 2017;265:814-20. [Crossref] [PubMed]
- Vanni G, Materazzo M, Pellicciaro M, et al. Breast Cancer and COVID-19: The Effect of Fear on Patients' Decision-making Process. In Vivo 2020;34:1651-9. [Crossref] [PubMed]
- Gaitanidis A, Alevizakos M, Tsalikidis C, et al. Refusal of Cancer-Directed Surgery by Breast Cancer Patients: Risk Factors and Survival Outcomes. Clin Breast Cancer 2018;18:e469-76. [Crossref] [PubMed]
- Kronenfeld JP, Crystal JS, Ryon EL, et al. Clinical Outcomes for Primary and Radiation-Associated Angiosarcoma of the Breast with Multimodal Treatment: Long-Term Survival Is Achievable. Cancers (Basel) 2021;13:3814. [Crossref] [PubMed]
- Heinhuis KM, IJzerman NS, van der Graaf WTA, et al. Neoadjuvant Systemic Treatment of Primary Angiosarcoma. Cancers (Basel) 2020;12:2251. [Crossref] [PubMed]
- Kunkiel M, Maczkiewicz M, Jagiełło-Gruszfeld A, et al. Primary angiosarcoma of the breast-series of 11 consecutive cases-a single-centre experience. Curr Oncol 2018;25:e50-3. [Crossref] [PubMed]
Cite this article as: Masanam MK, Merritt C, Ilagan C, Greenwalt IT, Tousimis EA. Primary angiosarcoma of the breast: a case report. AME Surg J 2023;3:18.


