
Biomarker status conversion in locoregional breast cancer recurrence in a patient who had achieved pathologic complete response: a case report
Introduction
Breast cancer is the second leading cause of cancer diagnoses across the globe and the most prevalently diagnosed cancer amongst women. Breast cancer recurrence in patients can present as a challenging ordeal for both the patient and the treating medical team. Local recurrence involves recurrence in the ipsilateral chest wall, while regional recurrence involves the ipsilateral axillary, supraclavicular/infraclavicular, or internal mammary lymph nodes (1). Neoadjuvant and adjuvant therapy is integral in the treatment of locally advanced breast cancer and breast cancer recurrence. These therapies are selected based on the subtype and biomarker status of a breast cancer. In cases of breast cancer recurrence, studies have indicated a lack of concordance in receptor status between recurrent and primary tumors (1,2). Breast cancer recurrence with phenotypical biomarker change is not a common occurrence but is documented in the medical literature in a wide range varying between 10% and 35–40% (3-7).
We present a rare case of locoregional recurrence of breast cancer with biomarker phenotype conversion in a patient who had achieved pathologic complete response (pCR) after nipple-sparing mastectomy and sentinel lymph node biopsy. We present the following case in accordance with the CARE reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-21-115/rc).
Case presentation
A 38-year-old female presented to our clinic for evaluation of a new left breast mass. She is otherwise healthy, gravida 3 para 2, has no family history of breast cancer, and is not of Ashkenazi Jewish ancestry. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the consent is available by the journal editorial office.
Of importance is her history of invasive ductal carcinoma in the upper outer quadrant of the left breast diagnosed one year prior. She initially presented to her gynecologist for evaluation of a palpable mass of the left breast that was biopsied (Figure 1). Pathology revealed a poorly differentiated invasive ductal carcinoma, estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, HER2/neu-negative, and Ki-67 50% (Figures 1,2). Fluorescent in situ hybridization (FISH) analysis confirmed negative HER2 gene amplification, group 4, and concurrent immunohistochemistry (IHC) score 0. Specifically, HER2/CEP17 ratio was 1.89, the average HER2 signals were 4.47 and the average CEP17 signals were 2.37. She underwent additional workup and a 2.3 cm mass abutting the left pectoralis muscle without evidence of invasion and a benign-appearing 0.8 cm left axillary lymph node was noted on MRI, Figure 3. Staging scans were negative for distant metastasis, and genetic testing was negative for any pathogenic mutations in any of the genes evaluated. For her cT2cN0M0 clinical stage IIA breast cancer, she underwent neoadjuvant chemotherapy with dose-dense cyclophosphamide and doxorubicin followed by Taxol (ddAC-T). Six months after her diagnosis, she underwent a bilateral nipple-sparing mastectomy and left sentinel lymph node biopsy with immediate implant-based reconstruction. Final pathology revealed no residual malignancy, no ductal carcinoma in-situ (DCIS), and the margins were negative. One sentinel node was retrieved and was negative for carcinoma, ypT0yN0.
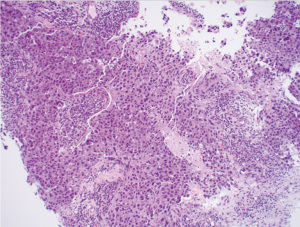
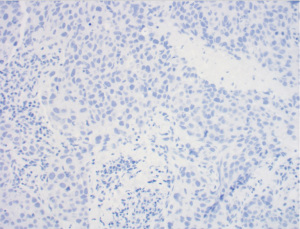

Four months after surgery, she noted a mass in the left upper outer chest wall that was increasing in size. The mass was biopsied and revealed a poorly differentiated invasive ductal carcinoma, ER-negative, PR-negative, HER2-positive by FISH. This was noted to be different from her original cancer pathology, which was HER2-negative. Moreover, the antibody clones for HER2 in the primary and recurrence tumors were dissimilar. In the primary tumor, the 4B5 rabbit monoclonal pathway from Ventana was observed. However, the antibody clone in the recurrence was Dako Herceptest Dxtm kit (link)/(Dako, Santa Clara, CA, USA). There was no lymphovascular invasion. Her CT scan imaging revealed a 4 cm mass in the left breast with axillary lymphadenopathy. She began chemotherapy with Taxotere + Carboplatin + Herceptin+ Perjeta (TCHP) and underwent therapy with 4 cycles; however the mass continued to clinically progress (Figure 4), growing in size, Figure 4A. She was changed to Kadcyla and underwent 2 cycles and continued to have clinical progression. The exam after her 2nd round of Kadcyla demonstrated her mass to measure 6 cm with ulceration and bleeding, Figure 4B. About 1 week later, the mass had worsening ulceration with exposed tumor, Figure 4C.

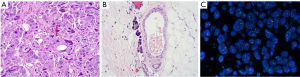
After a multidisciplinary discussion, the patient underwent an aggressive left chest wall wide local excision and axillary lymph node dissection. At the time of surgery, she was found to have tumor erosion into her pectoralis major and bulky lymphadenopathy during axillary dissection. She did well and was observed overnight and discharged the following day. Her pathology (Figure 5) after surgery revealed poorly differentiated recurrent invasive ductal carcinoma (Figure 5A) involving the adipose tissue and extending into the overlying skin with ulceration and involving the breast implant capsule. Lymphovascular invasion is present, Figure 5B. Her skin, soft tissue, and muscle margins were negative for malignancy. She had 21 of 25 axillary lymph nodes positive for metastatic carcinoma with extranodal involvement. Chest wall recurrence revealed an invasive ductal carcinoma which was HER2/neu equivocal by IHC, score of 2+ and positive by FISH (Figure 5C). An overview of the locoregional recurrence timeline can be found in Figure 6.
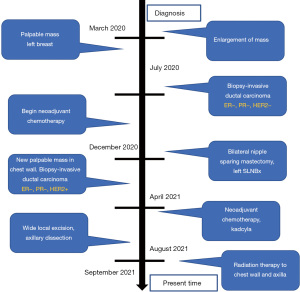
The patient felt overwhelmed at first, given a short time to recurrence. She was emotional with the new cancer diagnosis and her progression on chemotherapy. Ultimately a multidisciplinary approach was taken for her care, and she will continue to follow-up with our team.
Discussion
Breast cancer recurrence after a pCR is not common. In a retrospective study of patients who received anthracycline-based neoadjuvant therapy, recurrence rate after pCR was 7.1% (8). A similar study of patients who received taxane-based chemotherapy, found the recurrence rate was 13.6% (8). In a retrospective review by Chaudry et al. (9), a younger age ≤50 and locally advanced stage IIIB and IIIC breast cancer were associated with an increased risk of developing distant metastasis. HER2 positive disease and axillary node involvement at time of surgery have also been found to be independent predictors of recurrence after pCR and neoadjuvant therapy (8,10). The patient in this case is under age 50, however her primary disease was clinical stage IIA and she did not have axillary node involvement at the time of her initial surgery where she was found to have pCR. The notion of HER2 positive disease as an independent risk factor for recurrence after pCR is noteworthy and important to her case.
The patient in this case displayed tumor phenotype discordance in HER2 expression from primary to recurrent tumor. Her primary tumor was an invasive ductal carcinoma triple ER, PR, and HER2 negative, IHC score of 0. Her chest wall recurrence was an invasive ductal carcinoma that is HER2/neu equivocal by IHC, score of 2+ and positive by FISH, see Figure 5C. These results were verified by 2 observers.
The gain in HER2 biomarker positivity, as seen in our patient, raises an interesting question as to the reason for her biomarker status conversion. The common questions of preanalytical and analytical variability have to be asked, but we must also consider the phenomenon of tumor heterogenicity, and the impact of previous treatments (3). The possibility of a small foci of a tumor clone at the time of the primary breast cancer also exists.
Pre-analytical variability refers to the tissue handling before fixation and to the quality and length of fixation. Analytical variability refers to the antigen retrieval methods, staining method, and scoring (4). The use of IHC to detect biomarkers in breast cancer is well-established, however there is intrinsic subjectivity of the results. In regards to HER2 expression, IHC evaluates overexpression of the receptor protein at the surface of the cells whereas FISH evaluates the status of the HER2 gene in the nucleus (11). IHC staining discrepancy of HER2 ranges from 1 to greater than 50% and gene amplification heterogeneity in HER2 can range between from 5% to 30% (12).
There have been great efforts in the standardization and reproducibility in the pathological evaluation of breast cancer specimens such as reporting protocols by the College of American Pathologists (CAP) (13). However, 100% accuracy is not able to be achieved all the time. Performing simultaneous repeated receptor measurement on the original primary tumor specimen and the repeat biopsy has been proposed to minimize some of the analytical variability but it cannot adjust for all the preanalytical variables (3).
Intratumor heterogenicity refers to variability in different areas of a tumor (spatial) or to tumor progression over time (temporal) (12). Variability in the expression of biomarkers can lead to problems in interpretation and cause discordant results. In a retrospective study of 119 patients with breast cancer recurrence by Dieci et al. (2), 27 of 119 patients (22.7%) had a change in tumor biological phenotype determined by discordance; 98 patients had a HER2 negative receptor status at initial diagnosis of their primary tumor. Ten of those 98 (10.2%) however, gained HER2 positivity at the time of their recurrent cancer.
Discordance between a primary breast cancer and a breast cancer at recurrence has prognostic value. Patients who maintained or did not have a phenotypical marker change at cancer recurrence, had better outcomes in regards to their post recurrence survival (PRS) and overall survival (OS). They had a median of 51 versus 29 months for PRS and 119.2 versus 68 months for OS (2). In the same study, the change in a tumor phenotype at recurrence, particularly the loss of ER, PR, and/or HER2 expression was associated with a shorter PRS and OS. The poorest PRS and OS phenotype has been found in the patient population whose phenotype turned into a triple negative at recurrence. When compared to patients who had concordant markers at recurrence, the triple negative group had a median of 27 versus 51 months for PRS and 59.3 versus 119.2 months for OS (2).
The possibility of a small focus of a tumor clone at the time of the primary breast cancer should also be considered in this patient. Various cell clones can segregate in different areas of a tumor or scatter and intermingle within the same area (12). Small genomic changes such as mutations and alterations in the expression of individual genes can also occur during the evolution of a tumor (14). These alterations can have an effect on many processes including treatment response.
Surgical options, in a patient who previously had a mastectomy and presents with recurrent and aggressive disease includes wide local excision, as was performed on this patient. She previously had a nipple sparing mastectomy with direct to implant reconstruction. Nipple sparing mastectomy has been associated with a low recurrence rate. In a single institutional study of 322 patients undergoing 588 nipple sparing mastectomies, the recurrence rate was 3.1% (14); 60% of the recurrences were locoregional in the skin or chest wall and none of them occurred in the preserved nipple areola complex (14). In the above referenced study, of the 10 patients who had a recurrence, 50% (n=5) of them had a previous diagnosis of triple negative breast cancer and 1/3 of them had positive lymph nodes at the time of surgery (14). Our patient had skin and chest wall recurrence but no involvement of the nipple-areola complex. Nipple-sparing mastectomy remains an oncologically safe procedure with estimated disease-free survival rates of 95.7% and 92.3% at 3 and 5 years, respectively (15).
In triple negative breast cancer, the 5-year rate of locoregional recurrence after mastectomy in patients who have not undergone post-mastectomy radiation is 4.9% for local recurrence and 7.3% for regional recurrence (1). Our patient did not receive post mastectomy radiation after her initial operation as it was not indicated in her initial breast cancer presentation. The indications of post-mastectomy radiotherapy (PMRT) in patients who achieve pCR after neoadjuvant therapy have been studied but not yet widely established in the literature. In a recent systematic review by Shah et al. (16), pooled data analysis demonstrated a clear benefit of PMRT on locoregional recurrence in patients with clinical stage III and IV disease who had achieved pCR. There was no benefit to PMRT on locoregional recurrence to patients with clinical stage I and II disease, however, there are no large prospective randomized trials evaluating PMRT use with disease modification following neoadjuvant chemotherapy (16). Current National Comprehensive Cancer Network (NCCN) guidelines for the treatment of local and regional recurrence involves surgical resection radiation therapy when possible (17).
Chemotherapy is an important aspect of the treatment of locoregional recurrence. Five-year recurrence rates in patients who underwent neoadjuvant chemotherapy and achieved pCR range from 13% to 25% (8). Management for HER2+ breast cancer presently includes a first line taxane along with dual anti-HER2 blockade with pertuzumab and trastuzumab, followed by progression to trastuzumab emtansine (TDM-1) (18). Our patient received this protocol first through TCHP therapy followed by progression to Kadcyla. A paucity of studies investigate the efficacy of Kadcyla in patients previously treated with pertuzumab (13). In a retrospective cohort of 77 patients receiving Kadcyla after taxane plus dual anti-HER2 blockage, 27% of patients achieved objective response, 40% reached stable disease control (19). Recent studies have begun to evaluate the next chemotherapeutical approach in management of patients who continue to progress despite receiving established protocols. In a phase 2 registration study of trastuzumab-deruxtecan (Enhertu) of patients with HER2+ metastatic breast cancer who had undergone previous treatment with Kadcyla, overall response rate is 60.9% with a median duration of progression-free survival (PFS) of 16.4 months. Remarkably, efficacy rates were similar in patients who had received previous pertuzumab therapy as well (16). Our patient continued to have clinical progression of her HER2+ recurrent breast cancer on a TCHP regimen as well as on Kadcyla. This progression underscores the importance of further investigating multi-regimen therapy options in patients previously treated with targeted therapy.
Conclusions
This case report implies the importance of tissue diagnosis and biomarker analysis in breast cancer recurrence. A multi-faceted approach must be taken in patients with locoregional recurrence of breast cancer after neoadjuvant therapy with pCR. Local excision and axillary lymph node dissection followed by targeted neoadjuvant chemotherapy may provide a robust option to optimize PFS. Novel therapy options such as trastuzumab-deruxtecan may offer an additional approach in clinically progressed patients despite prior targeted therapy. Interobserver variability in the subjective scoring of biomarker results can contribute to differences in reproducibility; however, the phenomenon of intratumor heterogenicity must also be considered. Performing simultaneous repeated receptor measurement and analysis on the original primary tumor specimen and the repeat biopsy should always be performed when discordance is suspected. Medical therapies, such as endocrine and chemotherapy, should be adjusted promptly and accordingly to ensure the best chances of survival for the patient.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-21-115/rc
Peer Review File: Available at https://asj.amegroups.com/article/view/10.21037/asj-21-115/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-21-115/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhao X, Tang Y, Wang S, et al. Locoregional recurrence patterns in women with breast cancer who have not undergone post-mastectomy radiotherapy. Radiat Oncol 2020;15:212. [Crossref] [PubMed]
- Dieci MV, Barbieri E, Piacentini F, et al. Discordance in receptor status between primary and recurrent breast cancer has a prognostic impact: a single-institution analysis. Ann Oncol 2013;24:101-8. [Crossref] [PubMed]
- Pusztai L, Viale G, Kelly CM, et al. Estrogen and HER-2 receptor discordance between primary breast cancer and metastasis. Oncologist 2010;15:1164-8. [Crossref] [PubMed]
- Nguyen TH, Nguyen VH, Nguyen TL, et al. Evaluations of Biomarker Status Changes between Primary and Recurrent Tumor Tissue Samples in Breast Cancer Patients. Biomed Res Int 2019;2019:7391237. [Crossref] [PubMed]
- Shiino S, Kinoshita T, Yoshida M, et al. Prognostic Impact of Discordance in Hormone Receptor Status Between Primary and Recurrent Sites in Patients With Recurrent Breast Cancer. Clin Breast Cancer 2016;16:e133-40. [Crossref] [PubMed]
- Jin X, Jiang YZ, Chen S, et al. Prognostic value of receptor conversion after neoadjuvant chemotherapy in breast cancer patients: a prospective observational study. Oncotarget 2015;6:9600-11. [Crossref] [PubMed]
- Lu Y, Tong Y, Chen X, et al. Association of Biomarker Discrepancy and Treatment Decision, Disease Outcome in Recurrent/Metastatic Breast Cancer Patients. Front Oncol 2021;11:638619. [Crossref] [PubMed]
- Tanioka M, Shimizu C, Yonemori K, et al. Predictors of recurrence in breast cancer patients with a pathologic complete response after neoadjuvant chemotherapy. Br J Cancer 2010;103:297-302. [Crossref] [PubMed]
- Chaudry M, Lei X, Gonzalez-Angulo AM, et al. Recurrence and survival among breast cancer patients achieving a pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat 2015;153:417-23. [Crossref] [PubMed]
- Asaoka M, Narui K, Suganuma N, et al. Clinical and pathological predictors of recurrence in breast cancer patients achieving pathological complete response to neoadjuvant chemotherapy. Eur J Surg Oncol 2019;45:2289-94. [Crossref] [PubMed]
- Zaha DC. Significance of immunohistochemistry in breast cancer. World J Clin Oncol 2014;5:382-92. [Crossref] [PubMed]
- Turashvili G, Brogi E. Tumor Heterogeneity in Breast Cancer. Front Med (Lausanne) 2017;4:227. [Crossref] [PubMed]
- Fitzgibbins PL, Connololy JL, Bose S, et al. Protocol for the examination of resection specimens from patient with invasive carcinoma of the breast. College of American Pethologists 2021.
- Margenthaler JA, Gan C, Yan Y, et al. Oncologic Safety and Outcomes in Patients Undergoing Nipple-Sparing Mastectomy. J Am Coll Surg 2020;230:535-41. [Crossref] [PubMed]
- Smith BL, Tang R, Rai U, et al. Oncologic Safety of Nipple-Sparing Mastectomy in Women with Breast Cancer. J Am Coll Surg 2017;225:361-5. [Crossref] [PubMed]
- Shah R, Hunter-Smith A, Botes A, et al. Does post mastectomy radiotherapy reduce loco-regional recurrence rates in all clinical stages of breast cancer following a complete pathological response to neoadjuvant chemotherapy? A systematic review and meta-analysis of the literature. Breast Cancer Management 2020;9:2. [Crossref]
- National Comprehensive Cancer Network. Breast cancer (version 8.2021). 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)†. Ann Oncol 2018;29:1634-57. [Crossref] [PubMed]
- Modi S, Saura C, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med 2020;382:610-21. [Crossref] [PubMed]
Cite this article as: Maini M, Sogunro O, Greenwalt I, Sidawy M. Biomarker status conversion in locoregional breast cancer recurrence in a patient who had achieved pathologic complete response: a case report. AME Surg J 2023;3:27.


