
Tree-on-sunset appearance of rectal neuroendocrine tumors, a different approach in narrow-band imaging characterization—a case report
Introduction
Neuroendocrine tumors (NETs) are a peculiar group of indolent neoplasms arising from neuroendocrine cells. They are found mainly in the gastrointestinal tract and respiratory system. Of the digestive system, the rectum is the second most frequent site affected after the small intestines (1). Rectal NETs constitute 1–2% of all colorectal malignancies and are found in 0.05–0.07% of screening colonoscopies (2,3). Although this is relatively insignificant, early detection is crucial as 80–90% of them measure less than 10 mm in size, are confined within the submucosal layer, and are low grade in nature (4-6). These characteristics make it favorable for endoscopic treatment with excellent outcomes.
Owing to the submucosal location of rectal NETs, the integrity of the overlying mucosal surface is pristine and unaffected. Thus, these neoplasms are traditionally chanced upon by a faint yellowish hue resulting from significant chromogranin deposition. The yellowish discoloration is not a pathognomonic finding as benign tumors such as lipoma, gastrointestinal lymphoma, and myoma may also share this feature and make it challenging to differentiate one from the other (7). A different approach is thus required to distinguish NETs from other benign pathologies, as treatment is necessary to prevent potential locoregional spread.
Recently, a study reported a novel honeycombing appearance on the mucosal surface of rectal NETs through narrow-band imaging (NBI) and suggested that this could aid differentiation from adenomatous polyps (8). Though this may be true, we must remember that some inflammatory lesions may also possess equivalent characteristics.
We wish to present a case study on rectal NETs and describe a separately unique NBI observation. In doing so, we trust that it will provide an additional tool for characterizing rectal NETs. We present the following case in accordance with the CARE reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-22-20/rc).
Case presentation
A 62-year-old lady with underlying functional dyspepsia and irritable bowel syndrome came to us for a routine surveillance colonoscopy for colorectal cancer. She did not have any constitutional symptoms aside from intermittent episodes of bloatedness and epigastric discomfort. Over the past 16 years, she has had two colonoscopies performed in our center, for which these were normal. Her last colonoscopy was in 2010, and due to an unpleasant experience, she decided to forgo her subsequent surveillance colonoscopy scheduled 5 years later.
She is a compliant patient who never failed to attend her clinic follow-ups and regularly takes her medication. Over the years, her symptoms gradually improved with counseling and a suitable medication regime. Unfortunately, with the COVID-19 pandemic and strict nationwide quarantine implementation, her symptoms worsened over the last two years. She became troubled with anxiety and manifested with recurrent heartburn, epigastric fullness, bloatedness, and non-specific abdominal cramps. Fast forward to her latest clinic follow-up in April 2022, she expressed voluntary interest in a repeat upper gastrointestinal endoscopy and colonoscopy without our prompting. Clinical examination and blood investigations were unremarkable. Seeing that there were no contraindications for endoscopic examination and her next colonoscopy was long overdue, we obtained her consent for both procedures.
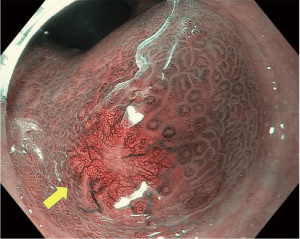
The upper endoscopy demonstrated Grade A reflux esophagitis with a mild hiatal hernia, while the colonoscopy revealed a subtle yellowish ‘bump’ within the lower rectum. The rectal lesion was a 10 mm flat, subepithelial lesion with normal, overlying mucosa (Figure 1). Characterization with NBI revealed fine capillaries traversing the surface (Figure 2). The microvascular patterns were less crowded towards the center of the lesion, and it resembled a tree-on-sunset appearance. We suspected this to be a NET and confirmed this through biopsies.


Following histological confirmation, we counseled her for a rectal endoscopic ultrasound (EUS) in 2 weeks to measure both; the size and depth of tumor invasion. We also planned an endoscopic resection in a similar setting should the EUS findings be favorable. The rectal EUS revealed a well-defined hypoechoic lesion measuring 10.2 mm by 5.1 mm within the submucosal layer of the rectal wall (Figure 3). The tumor was deemed suitable for endoscopic resection utilizing a modified endoscopic mucosal resection and ligating (EMR-L) device.

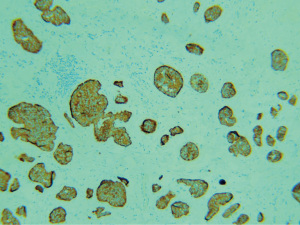
Firstly, lesion marking was done with forced argon plasma coagulation using the ERBE VIO 200D electrosurgical unit (ERBE, Tübingen, Germany) around the area of interest. Next, we perform a methylene blue-based injection around the tumor base to achieve an adequate submucosal lift (Figure 4). The endoscope was then withdrawn and fitted with the multi-band mucosectomy device (Duette, Cook Medical). After this, we identified the lesion based on the markings and applied endoscopic suctioning followed by band deployment to create a temporary pseudopolyp. We then used a dedicated 5 French mini hex snare to resect the pseudopolyp below the ligating band (Figure 5). The base on inspection was free from deep-muscle injury and bleeding. The procedure was a success, and our patient was discharged well on the same day. She experienced no adverse and unanticipated events and remained well during clinic follow-up two weeks and two months later. The subsequent histopathological assessment demonstrated multiple cribriform and nests of neuroendocrine cells with minimal atypia and no evidence of mitosis (Figure 6). Confirmation with immunohistochemistry evaluation revealed strong and diffusely positive staining for synaptophysin in keeping with rectal NETs (Figure 7). Further to this, the tumor was low-grade with a Ki-67 proliferative index of less than 3%. The resection margins were also clear from neoplastic tissue, deeming the endoscopic treatment absolute and curative.

All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In a recent article, Wang et al. subdivided the morphological appearance of rectal NETs on white light imaging based on the angle between the tumor’s edge and the mucosa’s bottom into four distinct types (9). They discovered that protuberant tumors (Type Ia and Ib) were the most encountered. Types II (flat lesions) and III (depressed or ulcerated center) were associated with a small risk of incomplete resection after propensity score matching and may require due diligence during endoscopic resection to achieve optimal outcomes (9). This morphological classification of rectal NETs is helpful as a guide in lesion recognition and subsequent selection of endoscopic techniques.
Nevertheless, we must be mindful that other subepithelial lesions can mimic rectal NETs, and relying on morphology alone is insufficient. When a high-confident diagnosis is not possible, referral to a tertiary center is preferable to performing biopsies. Unfortunately, taking biopsies may present several problems (10). For instance, obtaining inadequate and superficial specimens may result in normal colonic tissue leading to false reassurances and neglect for subsequent therapy and surveillance. On the flip side, performing larger and multiple biopsies with jumbo forceps lead to the inherent blurring of margins and submucosal fibrosis. Such drawbacks can make subsequent endoscopic resection a technically demanding procedure and lead to complications and suboptimal outcomes (10).
With the introduction of high-resolution colonoscopes, recent efforts in mucosal characterization of rectal NETs with magnification NBI revealed a newly described honeycomb pattern. The investigators observed this recurring pattern in their case series of thirteen patients and attributed it to the interplay between the engorged microsurface pits and the surrounding capillaries (8). Such description was not present in our case despite sharing many characteristics, including the same size, flat morphological appearance, and submucosal location. Instead, we coincidentally discovered fine, lacy capillaries traversing the lesion, resembling a tree-on-sunset appearance. This NBI finding was also present in a different patient of ours who was recently diagnosed with multiple gastric fundal NETs (Figure 8). Upon direct comparison, the tree-on-sunset pattern appears to be a separate entity from the honeycomb feature. Nevertheless, it could also be a precedent for becoming a proper honeycomb. Although we could not explain the development of the tree-on-sunset feature, we believe that it might share equal pathophysiological principles as the honeycomb appearance. Until more concrete data comes to light to address this limitation, there is no replacement for a meticulous colonoscopic examination.
Lately, rectal EUS with high-frequency ultrasonic probes has transformed the diagnostic landscape for rectal NETs. These instruments can localize tumors within the rectal wall with an accuracy of 90% to 100% and are indispensable for pre-resection investigation (11,12). In addition, rectal EUS can also measure the invasion depth of up to as little as 2 mm in diameter and guide subsequent choices of endoscopic treatment to achieve the best outcome (13,14). Ultimately, the aim is to obtain curative intent with the least possible radical approach. This objective is only possible when collective information on lesion size, morphological appearance, depth of invasion, and lymphovascular involvement is available (15,16).
Modifications to endoscopic mucosal resection (EMR) and the recent introduction of endoscopic submucosal dissection (ESD) have resulted in successful en bloc resection of rectal NETs through endoscopic means (17). The conventional EMR technique is a two-step procedure with submucosal injections to lift the lesion away from the muscularis propria, followed by snare excision and specimen retrieval. On the other hand, ESD involves four steps: lesion marking, submucosal lifting, mucosal incision, and submucosal dissection. The distinguishing factor between EMR and ESD is the latter’s ability in submucosal plane identification. Such capability grants microscopically margin-negative (R0) resection, the ideal scenario for curative intent. Unfortunately, ESD is a technically demanding procedure that requires high expertise that is not widely available. The costly and time-consuming operation makes it the least favored choice for small lesions, for which modified EMR is the better option (17). Of the various improvements described, the EMR-L technique shares similar technical success rates with ESD, ranging from 90% to 100% (17,18). The EMR-L approach permits deeper tissue grasping following endoscopic suctioning into the distal attachment cap, thus creating a pseudopolyp. A ligating band deployed then grips the base of the pseudopolyp created while sparing deeper muscle layers. The contraction property of the ligating bands is sufficient to entrap the submucosal layers but not strong enough to hold on to the muscularis propria, thus, allowing for safer resection and reduced perforation risk (19). The EMR-L technique is also easier to learn, more cost-effective, and quicker to perform when compared to ESD (20). Nevertheless, patients with rectal NETs measuring more than 10 mm are better candidates for ESD owing to the risk of incomplete resection with various improvised EMR techniques as the distal attachment cap is limited in tissue volume acquisition.
The EMR-L technique was the therapeutic option we selected for our patient as her tumor characteristics were favorable for the procedure. It was flat, measured 10 mm in size, low grade, and confined to the submucosal space. The en bloc resection was straightforward and deemed curative, thus dismissing the need for further investigation and radical treatment.
Conclusions
With the established improvements of modified endoscopic therapy to address resection of rectal NETs, our research focus must now shift towards lesion characterization. Relying on the gross morphological description is insufficient to distinguish it from other pathology. On the other hand, performing biopsies to obtain a diagnosis, as discussed, has its shortfalls. The discovery of the honeycomb mucosal pattern and our recent tree-on-sunset description from NBI may complement current observations and further improve lesion recognition, thus benefiting every endoscopist of all levels.
Acknowledgments
We wish to express our sincere gratitude and appreciation by acknowledging our team of gastrointestinal nurses who assisted us and provided valuable insights and feedback during the colonoscopy. They are AMO Valentine Lesley Philiminus, RN Rinah A/K Kijam, RN Hufaidah Awang Damit, RN Julianie Julian and RN Marianah Rampungan from Queen Elizabeth Hospital, Kota Kinabalu Sabah, Malaysia.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-22-20/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-22-20/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934-59. [Crossref] [PubMed]
- Taghavi S, Jayarajan SN, Powers BD, et al. Examining rectal carcinoids in the era of screening colonoscopy: a surveillance, epidemiology, and end results analysis. Dis Colon Rectum 2013;56:952-9. [Crossref] [PubMed]
- McDermott FD, Heeney A, Courtney D, et al. Rectal carcinoids: a systematic review. Surg Endosc 2014;28:2020-6. [Crossref] [PubMed]
- Kasuga A, Chino A, Uragami N, et al. Treatment strategy for rectal carcinoids: a clinicopathological analysis of 229 cases at a single cancer institution. J Gastroenterol Hepatol 2012;27:1801-7. [Crossref] [PubMed]
- de Mestier L, Brixi H, Gincul R, et al. Updating the management of patients with rectal neuroendocrine tumors. Endoscopy 2013;45:1039-46. [Crossref] [PubMed]
- Wang AY, Ahmad NA. Rectal carcinoids. Curr Opin Gastroenterol 2006;22:529-35. [Crossref] [PubMed]
- Lin CK, Chung CS, Chiang TH, et al. Detection of rectal neuroendocrine tumor during screening colonoscopy and its difference from colonic adenocarcinoma. Advances in Digestive Medicine 2017;4:99-104. [Crossref]
- Wang XY, Chai NL, Linghu EQ, et al. The outcomes of modified endoscopic mucosal resection and endoscopic submucosal dissection for the treatment of rectal neuroendocrine tumors and the value of endoscopic morphology classification in endoscopic resection. BMC Gastroenterol 2020;20:200. [Crossref] [PubMed]
- Lee SP, Sung IK, Kim JH, et al. The effect of preceding biopsy on complete endoscopic resection in rectal carcinoid tumor. J Korean Med Sci 2014;29:512-8. [Crossref] [PubMed]
- Park CH, Cheon JH, Kim JO, et al. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy 2011;43:790-5. [Crossref] [PubMed]
- Ishii N, Horiki N, Itoh T, et al. Endoscopic submucosal dissection and preoperative assessment with endoscopic ultrasonography for the treatment of rectal carcinoid tumors. Surg Endosc 2010;24:1413-9. [Crossref] [PubMed]
- Kobayashi K, Katsumata T, Yoshizawa S, et al. Indications of endoscopic polypectomy for rectal carcinoid tumors and clinical usefulness of endoscopic ultrasonography. Dis Colon Rectum 2005;48:285-91. [Crossref] [PubMed]
- Maione F, Chini A, Milone M, et al. Diagnosis and Management of Rectal Neuroendocrine Tumors (NETs). Diagnostics (Basel) 2021;11:771. [Crossref] [PubMed]
- Kim BN, Sohn DK, Hong CW, et al. Atypical endoscopic features can be associated with metastasis in rectal carcinoid tumors. Surg Endosc 2008;22:1992-6. [Crossref] [PubMed]
- Shim KN, Yang SK, Myung SJ, et al. Atypical endoscopic features of rectal carcinoids. Endoscopy 2004;36:313-6. [Crossref] [PubMed]
- Lee DS, Jeon SW, Park SY, et al. The feasibility of endoscopic submucosal dissection for rectal carcinoid tumors: comparison with endoscopic mucosal resection. Endoscopy 2010;42:647-51. [Crossref] [PubMed]
- Choi CW, Kang DH, Kim HW, et al. Comparison of endoscopic resection therapies for rectal carcinoid tumor: endoscopic submucosal dissection versus endoscopic mucosal resection using band ligation. J Clin Gastroenterol 2013;47:432-6. [Crossref] [PubMed]
- Alzoubaidi D, Graham D, Bassett P, et al. Comparison of two multiband mucosectomy devices for endoscopic resection of Barrett's esophagus-related neoplasia. Surg Endosc 2019;33:3665-72. [Crossref] [PubMed]
- Yang DH, Park Y, Park SH, et al. Cap-assisted EMR for rectal neuroendocrine tumors: comparisons with conventional EMR and endoscopic submucosal dissection (with videos). Gastrointest Endosc 2016;83:1015-22; quiz 1023-.e6.
Cite this article as: Chiam KH, Bala Krishnan A, Sriram N, Muthukaruppan R. Tree-on-sunset appearance of rectal neuroendocrine tumors, a different approach in narrow-band imaging characterization—a case report. AME Surg J 2023;3:29.




