
The most beneficial options for patients with arrhythmia and concomitant structural heart disease: a review
Introduction
The worldwide prevalence of atrial fibrillation (AF) is increasing (1), with an estimated 37 to 46 million people affected (2,3). Risk factors contributing to AF include increased age, diabetes, obesity, chronic obstructive pulmonary disease, heart failure, coronary artery disease, obstructive sleep apnea, alcohol consumption, physical inactivity, and smoking (4,5). In addition to being risk factors for AF, these are also common comorbidities found in cardiac surgery patients making them a fundamentally high-risk population for AF. These comorbidities combined with structural changes in the atria related to valvular disease and left ventricular dysfunction also support screening diligently for AF before patients go to the operating room.
The gravitas of untreated AF cannot be understated, with the most devastating outcomes being stroke, heart failure, and death. AF carries a three- to five-fold increase in stroke (6), and the severity of AF-associated strokes is worse than those occurring in the absence of AF (7). AF and heart failure often co-mingle, with AF contributing to left ventricular (LV) dysfunction leading to heart failure. Recent histopathologic evidence reveals that AF adversely affects LV myocyte function independent of heart rate (8). Thus, rate control is an inadequate defense against LV damage due to AF. Using Framingham Heart study data, Lubitz et al. reported that two-thirds of patients with AF experienced stroke, heart failure, or mortality within a decade of their first diagnosed episode of AF (9). In addition, AF symptoms are detrimental to quality-of-life (QOL) due to exercise intolerance, depression, and anxiety. The ORBIT-AF registry found that higher burden of AF symptoms correlated with reduced QOL and increased hospitalization rates, independent of major morbidity and mortality (10).
Healthcare resource utilization and productivity are strained by AF. Based on propensity score-matched analysis, patients with AF have significantly greater likelihoods of all-cause hospitalization, cardiovascular-related hospitalizations, and death during hospitalization (11). This translates into an estimated $8,000 extra cost per patient and up to $26 billion extra direct and indirect costs from AF per year. An analysis of the GARFIELD-AF registry found that AF accounted for an estimated €18 billion in healthcare and mortality-related costs in France, Germany, Italy, Spain and the United Kingdom (12). Clearly, the opportunity to detect and treat AF at the time of cardiac surgery should not be missed, due to its burden on individual patients and healthcare at large.
Recognizing the risk of untreated AF, and the variety of surgical approaches attempting to treat concomitant AF, the objective of this perspective is to offer a rationale for the most beneficial options. A discussion of relevant literature, and critical assessment of the advantages and limitations of different techniques are provided.
Implications of AF in patients with structural heart disease
Although the prevalence of AF varies among structural heart disease conditions, based on analysis of the Society of Thoracic Surgeons (STS) database, approximately 13% of patients undergoing cardiac operations have pre-operative AF (13). It is reasonable to assume the percentage is higher given the likelihood of undetected preoperative AF. Categorical rates of pre-operative AF among cardiac surgeries are approximately 30% for mitral valve (MV) surgery, 18% for isolated aortic valve replacement (AVR) (14), and 6% for coronary artery bypass graft (CABG) (15,16). With age comes more AF, and a study of the Medicare database by McCarthy et al. revealed pre-operative AF rates are 62% with MV surgery, 33% for AV surgery, and 20% of CABG (17).
Structural heart disease contributes to development of AF by increasing left atrial pressure leading to left atrial enlargement (18). Subsequently, chronic atrial stretch induces fibrosis and electroanatomic remodeling which initiate and perpetuate AF. Onset of AF may predate structural heart disease and thus have its genesis in medical comorbidities and/or genetic predisposition. In fact, preexisting AF can be the driver of valvular regurgitation for either or both atrioventricular valves. AF-related atriopathy and atrial expansion produces atrio-annular enlargement and insufficiency of the atrioventricular valves (19). Furthermore, tachycardic and non-tachycardic LV cardiomyopathy results from AF leading to LV remodeling, elevated LV end diastolic pressure, mitral regurgitation and pulmonary hypertension with subsequent elevated right sided pressures and tricuspid regurgitation (20).
The presence of pre-operative AF increases morbidity and death. Several studies evaluating matched cohorts of patients with pre-operative AF compared to those in sinus rhythm undergoing concomitant CABG and/or valve procedures had the same finding (21-24). The implications of pre-procedural AF do not disappear when the intervention is switched to transcatheter. In the PARTNER and PARTNER 2A trials, intermediate- and high-risk patients with baseline AF who underwent transcatheter aortic valve replacement (TAVR) experienced increased one- and two-year mortality rates compared to those in sinus rhythm (25,26). A substantial proportion of patients undergoing transcatheter mitral valve repair (TMVR) have pre-existing AF, with some studies reporting up to 67% prevalence (27). Meta-analyses have found increased risks for mortality, bleeding, and hospitalization with pre-operative AF in TMVR patients (28,29). The lifelong risks of AF and evidence supporting its treatment at the time of cardiac surgery are so compelling that guidelines of the European Association for Cardio-Thoracic Surgery (EACTS) (5), STS (30) and American Association for Thoracic Surgery (AATS) (31) all recommend its concomitant treatment.
Surgical arrythmia management
The Cox-Maze is a concept whereby transmural lesions are created in a pattern isolating the posterior wall of the left atrium and transecting pathways in both atria identified through electrophysiologic studies as mechanisms for sustaining atrial flutter and fibrillation, while leaving the corridor of conduction from the sino-atrial node to the atrio-ventricular node intact (Figure 1). The cut-and-sew Cox-Maze established the highest level of efficacy achievable in AF treatment. After performing the first Cox-Maze in 1987 (32), Dr. Cox and colleagues made changes to the position of lesions arriving at the Cox-Maze III, which serves as the template for all current surgical AF therapy. Even with the third iteration, the cut-and-sew operation was too technically complex for widespread adoption. In 2002, the Cox-Maze IV was introduced by Dr. Gaynor and colleagues, using ablative energy devices to create nearly all of the lesions (33). The goal of using radiofrequency or cryothermal energy is the same as a knife: transmural lesions. In the Cox-Maze IV, a bipolar radiofrequency clamp is used to create most of the lesions, while cryoablation probes are applied for those crossing the coronary sinus and annuli (Figure 1). The atriotomies are incorporated into the lesion set. With regulatory approval of the bipolar clamp to treat concomitant persistent AF came the opportunity to teach the operation globally (34). The Cox-Maze IV performed with radiofrequency and cryothermal energy became the gold standard for concomitant surgical ablation to treat AF (31).
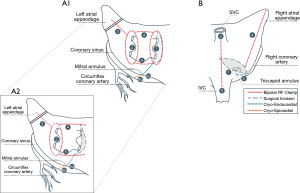
Cryothermal energy is advantageous when ablating across the tricuspid and MV annuli because it does not denature collagen. Properly applied, cell death is assured, and animal studies have shown excellent transmurality can be achieved with cryoablation (35,36). In fact, Cox described a minimally invasive approach to Cox-Maze III with cryoablation in 1996 (37). Retrospective studies have compared safety and effectiveness of surgical ablation performed exclusively with cryoablation to that performed with combined radiofrequency and cryothermal energy (38). One study found similar safety and effectiveness (38), while another suggested higher rates of sinus rhythm off anti-arrhythmic drugs (AADs) and fewer strokes associated with cryothermal energy (39), but lacked clarity on the lesion set. Currently there is an ongoing investigational device exemption trial to evaluate safety and effectiveness of cryothermal-only Cox-Maze III ablation concomitant with cardiac surgery for treatment of persistent and longstanding persistent AF (NCT03732794).
Implications of treating AF during concomitant open-heart surgery
Clinical trials, including randomized controlled (40), investigational device exemption (34), and post-approval studies (41), have all revealed favorable rhythm outcomes with concomitant Cox-Maze IV. Now that nearly 20 years passed since the first published series of Cox-Maze IV procedures, long-term rhythm outcomes data has accumulated. There is variability in data collection methods and technique for surgical ablation creating a less than perfect understanding of the implications of different approaches, but clearly rhythm restoration can be accomplished during concomitant open-heart surgery (Table 1). Khiabani et al. reported on 10-year outcomes of surgical ablation at a single-center (42). Of the 853 patients, most patients had ablation via sternotomy, most procedures were performed concomitantly with a cardiac surgical procedure, and most patients received a bi-atrial Cox-Maze IV. With concomitant surgical ablation, freedom from atrial tachyarrhythmias at 5 years was 82.7% in 179 patients and 10 years was 75.4% in 65 patients. Off AADs, these rates were 70.9% and 61.5%, respectively. The authors found no differences in outcomes between sternotomy and right mini-thoracotomy nor between concomitant and standalone procedures. Ad et al. reported rhythm outcomes after Cox-Maze III/IV procedures during MV procedures, 89% of which were performed via sternotomy (43). At 5 years (n=158) and 7 years (n=80) since the procedure, 80% and 66% of patients were in sinus rhythm, and 69% and 55% were in sinus rhythm off AADs, respectively.
Table 1
| Study | Index procedure | Lesion set | Time-point | Freedom from atrial arrhythmias or in SR | Freedom from atrial arrhythmias or in SR off AADs | Overall survival | Freedom from stroke |
|---|---|---|---|---|---|---|---|
| McCarthy et al. 2022, (41) | Isolated valve (36%) CABG/double valve (7%) Double valve (23%) Isolated CABG (18%) CABG/valve (16%) |
Biatrial CMIV: 94.5% | 3 years | 73.7% | 64.3% | 81.7% | 95.6% |
| Khiabani et al. 2022, (42) | Mitral ± tricuspid valve (51%) CABG ± mitral valve (20%) Aortic valve ± CABG (16%) Aortic ± mitral valve (4%) Other (9%) |
Biatrial CMIV: 89.7% | 10 years | 75.4%* | 61.5%* | 86%* | NR |
| Ad et al. 2018, (43) | Isolated mitral valve (46%) Mitral valve + other (54%) |
CMIV: 45% CMIII (Cryo): 55% |
7 years | 66% | 55% | 77% | 96.6% |
*, outcomes for concomitant ablation procedures, excludes standalone ablation. SR, sinus rhythm; AADs, anti-arrhythmic drugs; CABG, coronary artery bypass graft; CM, Cox-Maze; CMIII, Cox-Maze III; CMIV, Cox-Maze IV; NR, not reported.
Perhaps most importantly, the effect of concomitant surgical ablation on late mortality has also been investigated (Table 2). In a propensity-score matched comparison of cardiac surgery patients with AF who underwent Cox-Maze ablation compared to those with untreated AF, there was a significantly higher 10-year overall survival rate with Cox-Maze IV ablation compared to no AF treatment (adjusted HR 0.47) (44). The 10-year survival with Cox-Maze treatment was similar to survival of patients who did not have pre-operative AF (adjusted HR 1.03). Three studies using a large Polish registry have also reported a benefit of surgical AF ablation on late mortality in patients undergoing cardiac surgery using propensity-score matched groups (45). The investigators found significantly reduced risks of late-term mortality when surgical AF ablation was performed concomitant with isolated CABG (HR 0.67, P=0.008, median 4.3 years follow-up) (46), MV surgery (HR 0.82, P=0.011, median 5 years follow-up) (45), and in a population who had isolated or combined CABG surgery (HR 0.74, P=0.036, median 4 years follow-up) (47). Similarly, an analysis of the Northern New England Cardiovascular Disease Study Group Cardiac Registry demonstrated the risk-adjusted HR for 5-year survival with surgical AF ablation concomitant with cardiac surgery (CABG, valve, or combination) was 0.69 compared to cardiac surgery without AF ablation, with mean 2.6 years of follow-up for survival (range, 0–7.6 years) (48). In effect, observational data from registry and single-center studies support a long-term survival benefit of surgical ablation concomitant with cardiac surgery in patients with AF.
Table 2
| Study | Concomitant surgery type | Source population | Study period | Follow-up time, median (IQR) or mean ± SD years | Reported increased peri-operative outcomes in SA group | Adjusted long-term survival rate benefit with SA |
|---|---|---|---|---|---|---|
| Musharbash et al. 2018, (44) | CABG, Aortic valve, mitral valve, tricuspid valve | Single-center | 2001–2016 | SA: 4.2±3.4 No SA: 3.8±3.8 |
Cross clamp time, 97 vs. 87 min (P<0.001) CBP time, 193 vs. 132 min (P<0.001) Pneumonia, 11% vs. 7% (P=0.045) Pacemaker implant: 12% vs. 5% (P=0.002) ICU LOS, median 3.6 vs. 2.2 days (P<0.001) Hospital LOS, median 11 vs. 8 days (P<0.001) |
53%, P=0.014 |
| Suwalski et al. 2019, (45) | Mitral valve | National registry | 2006–2017 | 5 (1.9–7.9) | None | 18%, P=0.01 |
| Suwalski et al. 2020, (46) | Isolated CABG | National registry | 2006–2018 | 4.3 (1.7–7.4) | None | 33%, P=0.008 |
| Kowalewski et al. 2020, (47) | Isolated/combined CABG | National registry | 2006–2019 | 4 (1.3–6.8) | Respiratory failure 8.1% vs. 4.6% (P=0.04) | 26%, P=0.036 |
| Iribarne et al. 2019, (48) | CABG, valve, CABG/valve | Regional registry | 2008–2015 | Mean 2.6 (range, 0–7.6 years) | Cross clamp time, 92.9 vs. 86.3 min (P=0.037) CPB time, 137.8 vs. 122.7 min (P<0.001) |
31%, P=0.013 |
SA, surgical ablation; IQR, interquartile range; SD, standard deviation; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; ICU, intensive care unit; LOS, length of stay.
Database analysis reveals that surgical ablation is predominately performed during MV procedures, and less commonly with isolated CABG and AVR procedures. One obvious explanation is the need to enter the left atrium. While non-atriotomy left atrial and bi-atrial lesion sets performed during concomitant open non-MV surgeries have been described, published data is currently limited to technique (49,50). To compare the outcomes of performing surgical Cox-Maze concomitantly with a MV versus a concomitant non-MV procedure, Ad et al. performed propensity score matching to identify two balanced cohorts of 164 patients each (51). Peri-operative outcomes (incidence of stroke, prolonged ventilation, renal failure requiring temporary dialysis, pacemaker implantation, and readmission within 30 days), length of hospital stay (median 7 days for each group, P=0.843), and 30-day operative mortality rates (1.8% in each group, P>0.999) were similar between groups. Furthermore, the annual proportions of patients in sinus rhythm irrespective of AADs were similar out to 5 years after the procedure. The proportion of patients in sinus rhythm was 80% at 5 years after Cox-Maze with MV surgery and 72% with non-MV surgery (P=0.303). Off AADs, 68% of patients who received Cox-Maze with MV surgery and 63% who received Cox-Maze with non-MV surgery were in sinus rhythm (P=0.492).
In addition to single- and multi-center studies evaluating the effectiveness of surgical ablation to restore normal sinus rhythm and reduce AAD use, studies have leveraged national databases to evaluate clinical outcomes of surgically treating AF during concomitant open-heart surgery. Based on a propensity score matched analysis of the STS database between 2011 and 2014, Badhwar et al. found concomitant surgical ablation during CABG and valve procedures reduced the relative risk (RR) of 30-day mortality (RR 0.92, 95% CI: 0.85–0.99) and stroke (RR 0.84, 95% CI: 0.74–0.94) compared to patients who did not undergo surgical AF ablation, though RRs of renal failure (1.12, 95% CI: 1.03–1.22) and pacemaker implantation (RR 1.33, 95% CI 1.24–1.43) were increased (13). Another propensity-score matched analysis of the STS database reported that surgical ablation with isolated aortic valve procedures did not add 30-day operative mortality or post-operative stroke risk compared to no ablation, however pacemaker implantation was increased from 5% to 6.8% (14). As the authors point out, this early essentially neutral finding should be framed in the context of the known long-term benefits of concomitant AF treatment.
Guidelines for concomitant treatment of AF during cardiac surgery
Based on available data in 2017, the STS gave surgical ablation a Class I recommendation based on Level A evidence when performed concomitantly with MV repair/replacement and a Class I recommendation based on Level B evidence when performed concomitantly with isolated AVR, isolated CABG, or AVR/CABG procedures (30). While AATS has given a Class IIA recommendation to the lack of added peri-operative and long-term morbidity and mortality, reduction of AF symptoms, and improvement in QOL associated with concomitant surgical ablation (31), they make a Class I recommendation for the observed reduction in operative mortality. The 2020 European Society of Cardiology (ESC)/EACTS guidelines gave surgical ablation a Class IIA (Level A evidence) recommendation that it should be considered with concomitant cardiac surgery (5).
Undertreatment of concomitant AF in the presence of structural heart disease
Taken together, based on published evidence (I) untreated AF in the context of structural heart disease portends poorer clinical outcomes; (II) concomitant surgical AF ablation restores normal sinus rhythm and reduces AAD use in most patients; and (III) consensus guidelines support concomitant surgical AF ablation. Notwithstanding, AF remains undertreated in the cardiac surgery patients. Based on the STS database, slightly less than half (48%) of patients with AF received surgical ablation at the time of concomitant cardiac surgery for structural heart disease between 2011 and 2014 and it was dominated by mitral surgery (13). In the Medicare population, the proportion of patients with AF treated with concomitant surgical ablation was 22% in 2014 (17).
Minimally invasive cardiac surgery—do we have to give up on AF treatment?
As minimally invasive approaches have flourished, we lack a granular understanding of the frequency that AF is overlooked or inadequately treated as a consequence of the approach. When intervention for AF is undertaken, it may be anywhere on a spectrum from left atrial appendage (LAA) management alone, to a complete Cox-Maze. For mini-thoracotomy MV surgery, a Cox-Maze can and should be performed. Lee and colleagues described a minimally invasive Cox-Maze IV (52). Because the clamp cannot be applied to a cuff of atrial tissue excluding the left pulmonary veins (PVs), cryoablation lesions are made to join the roof and floor lines anterior to the left PVs. In their first report, all sixteen patients with 12-month follow-up were free from AF recurrence and 81% were off AADs. In later experience comparing MV surgery patients undergoing Cox-Maze IV via mini-thoracotomy (n=104) to sternotomy (n=252), they found no difference in freedom from atrial arrhythmias in the absence of AADs at 12 and 24 months (53). Continued similar effectiveness of performing Cox-Maze IV through sternotomy or right mini-thoracotomy was confirmed long-term (42).
As previously mentioned, as early as 1996, Dr. Cox reported cryo-ablation Cox-Maze via right min-thoracotomy. It remains a well described option during minimally invasive mitral surgery. Dr. Ad who participated in the earliest procedures and reports, along with colleagues focusing on complete cryo-lesion sets have demonstrated equivalence in rhythm outcome for the Cryo Cox-Maze III via sternotomy or mini-thoracotomy, and in comparison to the Cox-Maze IV (mainly radiofrequency with adjunct cryoablation) (39). Their reports include via right mini-thoracotomy on a fibrillating heart without cross clamping (54). Techniques for minimally invasive, robotic-assisted Cox-Maze ablation performed with cryothermal ablation have been published (55,56).
Minimally invasive aortic valve procedures are challenging for concomitant Cox-Maze. Non-atriotomy techniques such as performing pulmonary vein isolation (PVI) with or without additional left atrial lesions during concomitant AVR have been described via partial sternotomy (57,58) and right minithoracotomy (59), with some approaches permitting only epicardial ablation, which has inherent challenges to transmurality. In effect, surgical ablation during minimally invasive aortic valve surgery is currently limited without a standard approach supported by safety and effectiveness data.
With the advent of TAVR, patients who otherwise would be treated for both aortic stenosis and AF may undergo TAVR only. Recent literature reveals both the substantial prevalence of preoperative AF in patients undergoing TAVR and the increased incidence of adverse post-operative events, including stroke and death (28,29). The pattern resembles that of open cardiac surgery in relation to AF. As described above, eventually overwhelming evidence emerged to show AF should not go untreated when addressing structural heart disease, at least when surgical.
Cox-Maze: pattern and transmurality
It cannot be stressed enough that all the most successful series whether via sternotomy or minimally invasive replicate the Cox-Maze in both pattern and transmurality. The full, biatrial Cox-Maze IV lesion set is depicted in Figure 1. The Cox-Maze does not exist in a lesser version. In the absence of transmurality or with any lesion subtracted, it is not a Cox-Maze. Every lesion must be as transmural as a well wielded knife. Every lesion must anchor into (cross) another transmural lesion or nonconducting tissue. The coronary sinus must be ablated in direct apposition to the partner myocardial isthmus lesion (60).
Achieving lesion transmurality requires diligence and attention to detail. When the author asked Dr. Cox how they determined adequate time of freezing with cryothermy during the early experience, Dr. Cox replied that they waited to see freeze-through ice and then waited at least two minutes longer. Seeing ice form outside the tissue opposite the probe does not signal a transmural lethal heat extraction. It simply means the temperature has reached 0 ℃ and water vapor in the air is crystalizing. All the cells must be exposed to a lethal temperature below −30 ℃ for at least 30 seconds. The time versus depth relation of cryothermal lesions is well described (61). Because the time dependence varies with myocardial thickness, in the ongoing ICE-AFIB trial, atrial cryoablation lesions are created with a minimum freeze time of at least two minutes, with three minutes for the left atrium and two minutes for the right atrium.
The means of ensuring transmurality with bipolar radiofrequency has recently been substantially simplified. Khiabani et al. working in Dr. Damiano’s lab using freshly explanted human hearts demonstrated complete transmurality using the AtriCure Synergy clamp (62). They compared patterns of energy delivery and found that administering the radiofrequency energy through two complete cycles without opening the clamp resulted in transmurality regardless of the algorithm waveform. If a lesion set is performed without ensuring transmurality, it is not a Cox-Maze. With the strength of lesion quality established using repeat closed clamp technique, it is reasonable to use only the left atriotomy which connects the roof and floor lesions to complete the box on the right side of the posterior left atrium (Figure 1-A2).
Minimal procedural requirements for surgical ablation to treat AF
The best long-term evidence for return to normal sinus rhythm exists for the full Cox-Maze. The pathophysiology of concomitant AF differs among patients. Evidence suggests AF secondary to left sided cardiac disease is predominantly due to left atrial substrate changes (18). If true, then selective patients could be treated with left atrial lesions only. The counterbalance being the likelihood of right atrial disease developing when AF is persistent. Thus, there is a sliding scale of pathology and persistence that moves from left side only lesions being adequate to inadequate. Some will make the argument that right side lesions may be added later by an electrophysiologist (63), but it seems a steep trade-off for a few minutes of surgical treatment. Others claim the need for permanent pacemakers increases with right side lesions (40), but it rings hollow when examining the mechanism (64). Importantly, the AF triggers in left side structural heart disease are not as often restricted to the ostia of the PVs as for non-valvular AF, but instead are typically more broadly spread across the left atrial posterior wall (65,66). As such the minimal treatment for concomitant paroxysmal AF should be a complete posterior left atrial box.
PV isolation is indeed integral to the Cox-Maze IV, but only as components of a complete posterior box, which is prerequisite for any Cox-Maze (Figure 2). The original Cox-Maze IV published in 2004 included only an inferior (floor) line connecting the right and left PV encircling lesions (33). The superior (roof) line to complete a posterior wall box was later described by Voeller et al. Subsequent studies showed long-term freedom from atrial arrhythmias with or without AADs was significantly improved by complete posterior wall isolation (67). Absence of a posterior box lesion was predictive of atrial arrhythmia recurrence or AAD use at 1 year following ablation in multivariate analysis, with an odds ratio of 4.5 (68). Posterior wall isolation is also the premise behind hybrid epicardial-endocardial strategies for patients with stand-alone AF. The recent CONVERGE trial showed 68% freedom from atrial arrhythmias absent new/increased dose of AADs through 12 months in patients with non-paroxysmal AF using the minimally invasive hybrid convergent procedure to isolate the PVs and left atrial posterior wall (69). Seventy-four percent of patients had ≥90% AF burden reduction at 18 months. The significance is twofold. First, clearly isolation of the posterior wall is a cornerstone of treatment; second, limiting the intervention to the posterior wall limits success. While concomitant paroxysmal AF and perhaps some early concomitant persistent AF may be adequately addressed with left side only lesions, AF in patients with large right or left atria or long-standing AF should undergo a complete Cox-Maze.
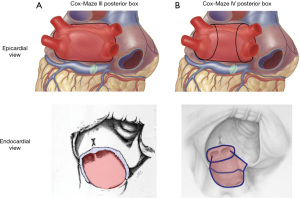
The scenarios for a posterior box only lesion set arise as a function of AF progression and surgeon comfort and perspective regarding added risk. The primary objective when addressing concomitant paroxysmal AF is exclusion of the triggers responsible for initiating the paroxysms, which reside largely in the posterior wall and PV orifices. Once persistent AF dominates the electrical behavior of the atria, the need to divide the pathways responsible for maintenance of AF progresses toward absolute. Given that the atria are an electrical syncytium, they both should be treated.
As the surgeon’s familiarity with the Cox-Maze increases, so does the probability of a complete Cox-Maze, and as the atrial pathology and AF burden diminish, the extent of the lesion set may fall. Undeniably the Cox-Maze will best address current AF pathophysiology and lessen the potential that further substrate changes will allow for its return under any circumstances. The reality is that we have little insight into the extent of atrial myopathy based simply on AF burden and duration (70). In fact, the time someone has experienced AF is not always clear. Nonetheless, allowance must be made to accommodate varying combinations of AF burden, operative complexity and surgeon expertise. For paroxysmal and early persistent AF, left side only lesion set is a reasonable option, especially in the absence of right heart pathology or pulmonary hypertension. Continued paring down of lesions will further sacrifice efficacy but again, may be considered in the context of a given combination of patient and surgeon. A poorly performed isthmus lesion, lacking transmurality or a coinciding coronary sinus lesion will provide substrate for very difficult to manage left atrial flutter. As such, the isthmus lesions are better left undone than done inadequately.
As described above, The CONVERGE trial demonstrated the efficacy of minimally invasive posterior wall ablation combined with percutaneous PV isolation to treat stand-alone persistent and long-standing persistent AF (69). The procedure mimics the electrical consequence of the box lesions included in the Cox-Maze. As would be predicted, the outcomes were less than a Cox-Maze, however, they were adequate to gain FDA approval as the only minimally invasive and/or percutaneous procedure to address long-standing persistent AF.
To provide a rapid option for a posterior encircling lesion including the posterior wall and PVs in a single device application, the EnCompass clamp* was designed for open chest concomitant operations. By sending large bipolar jaws through the transverse and oblique sinuses simultaneously, once positioned, closure of the clamp creates a single loop of transmural bipolar radiofrequency injury and applies the same algorithm as the Synergy clamp. In effect, in the same fashion as a cuff of left atrial tissue is isolated with the PVs during standard bipolar radiofrequency PVI, the EnCompass isolates the posterior left atrial wall along with the PVs. The EnCompass serves as a means of performing the box lesions without opening the left atrium, and thus lends itself to use when time is of the essence and/or surgeon preference includes not making a left atriotomy. Depending on the clinical scenario, it may have legitimacy for AF from paroxysmal to persistent long-standing, however effectiveness remains to be evaluated. When employing box only lesions, it behooves the surgeon to have established a patient management pathway that includes electrophysiologists who are willing to interrogate and add to the lesions over time if needed. In fact, the same can be said of any surgical ablation not amounting to a Cox-Maze. Equally important, the surgeon should never indicate a Cox-Maze was performed unless the entire biatrial Cox-Maze with isthmus and coronary sinus lesions was completed.
Evidence for management of the left atrial appendage
The Cox-Maze procedure includes exclusion or elimination of the LAA, as it is the predominant site of thrombus formation in AF and has been shown to have arrhythmogenic activity (71). In 1999, Cox et al. reported on the paucity of perioperative (0.7%) and strokes that occurred during long-term follow-up (0.4%, 3 months to 11.5 years) of 306 patients treated with surgical ablation (72). The low incidence of strokes was attributed to success achieving normal sinus rhythm. However, it was also hypothesized that removing the LAA played an important role, as not all patients maintained sinus rhythm. More recently, the landmark Left Atrial Appendage Occlusion Study (LAAOS) III trial showed that surgical occlusion of the LAA during concomitant cardiac surgical procedures in patients with pre-operative AF and CHA2DS2-VASC ≥2 significantly reduced ischemic stroke and systemic embolism, even with continued standard of care anticoagulation (73). Therefore, management of the LAA is an important component of surgical treatment of AF. The method of occlusion is critical to eliminate communication between the left atrium and LAA. Surgical suture ligation has proven most problematic. In LAAOS I, the incomplete closure rate was 72% and suture ligation was not permitted in the subsequent LAAOS II or LAAOS III trials (74). When follow up imaging has been performed to assess residual communication or stump >1 cm, substantial failure rates have been documented for internal suture closure and stapler exclusion as well (74-77). Diligence to ensure a well closed LAA is imperative, given incomplete LAA closure appears to confer greater risk of thrombus formation and thromboembolism (78-80).
Application of the AtriClip external LAA closure device during open sternotomy cardiac surgery has been studied with follow-up cardiac CT angiography and TEE at 3 months. In two clinical trials, 90% of study patients had follow-up imaging at 3 months, and complete closure without communication or residual pouch greater than 10 mm was documented in 99% of cases (81,82). Long-term CT angiography and TEE in trial and registry patients found 98% closure in 43 patients with a mean 7.1±0.8 years after clip placement (83). Of all studied modalities for surgical LAA management, the LAA clip demonstrates the highest efficacy.
The use of an epicardial clip to exclude the LAA during minimally invasive cryothermal Cox-Maze ablation with MV repair has also been described (84). Rhee et al. recently reported on LAA exclusion with an epicardial clip through minithoracotomy in 181 patients, of whom 81% had concomitant minimally invasive MV surgery and 84% had concomitant surgical ablation (85). In 103 patients with post-operative CT imaging, no residual flow between the left atrium and LAA was detected in any patient and 92% of patients had residual stump <10 mm.
Scenarios where surgical AF treatment may be inappropriate
Surgical scenarios exist during which concomitant surgical AF treatment should not be undertaken. Large left atrial size is a predictor of atrial tachyarrhythmia recurrence, with 8 cm suggested as a threshold leading to at least 50% recurrence rate (86). Patients with left atrial calcification are not candidates for surgical AF ablation. Furthermore, circumstances may arise in which the procedure is not safe to perform. Though multiple publications led to society guidelines determining the addition of surgical AF treatment does not increase risk, some combination of challenging patient characteristics, procedural complexity, and surgical expertise will influence how safe it is to proceed with concomitant AF treatment.
Ongoing management of AF and concomitant structural heart disease
AF patients presenting for cardiac surgery must be managed for the entirety of the cardiac pathophysiology. To unwind the spiral of pathophysiology, in addition to repairing the valves, the AF must be dealt with aggressively. Just as importantly, the treatment of AF for any patient does not end in the operating room. AF is a chronic progressive disease, and its return is hastened by inattentiveness to the medical conditions that conspire to induce it. Patients who are followed closely and treated for concomitant disorders are more likely to remain in rhythm (87).
Conclusions
Pre-operative AF in patients undergoing cardiac surgery and transcatheter structural heart procedures is associated with negative consequences compared to the absence of AF. Furthermore, matched comparisons demonstrate that cardiac surgery patients with AF who have surgical AF ablation have improved outcomes, without sacrificing peri-operative safety. The concomitant bi-atrial Cox-Maze is supported by the most robust evidence. The introduction of the Cox-Maze IV has led to widespread teaching and implementation. A Cox-Maze can also be accomplished with diligent application of cryothermy for all of the lesions. Depending on the composite of disease burden and the balance of surgeon skill and operative complexity, lesser lesions sets may be successfully employed. Every arrythmia surgeon should have insight into the progression of substrate modification and burden of AF in lesion set selection. A left atrial lesion set including posterior wall—PV box isolation should be prioritized, as well as complete LAA closure. When performing less than a biatrial Cox-Maze, the surgeon should not apply the label Cox-Maze. Lesser lesion sets should be well documented and electrophysiology colleagues enlisted for potential subsequent interventions. With increasing minimally invasive cardiac surgery and transcatheter procedures, the sequela of pre-operative AF must be weighed in decision making.
Acknowledgments
The AtriCure Isolator Synergy EnCompass Clamp and Guide System is intended to ablate cardiac tissue during surgery. Its safety and effectiveness for the treatment of atrial fibrillation have not been established. Kristen Plasseraud, PhD (AtriCure, Inc.) provided medical writing assistance under the direction of Marc Gerdisch.
Funding: Dr. Gerdisch receives research grants and/or consultant fees from AtriCure, ZimmerBiomet, CryoLife, Edwards Lifescience, and CorMatrix.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Anna Witkowska and Piotr Suwalski) for the series “Management of Cardiac Arrhythmia - Broadening the Horizons of Surgical Interventions” published in AME Surgical Journal. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-22-18/coif). The series “Management of Cardiac Arrhythmia - Broadening the Horizons of Surgical Interventions” was commissioned by the editorial office without any funding or sponsorship. MWG is an instructor for the Cox-Maze course (AtriCure, Inc.). He receives research grants and/or consultant fees from AtriCure, ZimmerBiomet, CryoLife, Edwards Lifescience, and CorMatrix. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 2015;386:154-62. [Crossref] [PubMed]
- Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke 2021;16:217-21. [Crossref] [PubMed]
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019;139:e56-e528. [Crossref] [PubMed]
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275-444. [Crossref] [PubMed]
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373-498. [Crossref] [PubMed]
- Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998;82:2N-9N. [Crossref] [PubMed]
- Miller PS, Andersson FL, Kalra L. Are cost benefits of anticoagulation for stroke prevention in atrial fibrillation underestimated? Stroke 2005;36:360-6. [Crossref] [PubMed]
- Pabel S, Knierim M, Stehle T, et al. Effects of Atrial Fibrillation on the Human Ventricle. Circ Res 2022;130:994-1010. [Crossref] [PubMed]
- Lubitz SA, Moser C, Sullivan L, et al. Atrial fibrillation patterns and risks of subsequent stroke, heart failure, or death in the community. J Am Heart Assoc 2013;2:e000126. [Crossref] [PubMed]
- Freeman JV, Simon DN, Go AS, et al. Association Between Atrial Fibrillation Symptoms, Quality of Life, and Patient Outcomes: Results From the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circ Cardiovasc Qual Outcomes 2015;8:393-402. [Crossref] [PubMed]
- Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes 2011;4:313-20. [Crossref] [PubMed]
- Mantovani L, Cossolino P, Angiolella L, et al. The burden of atrial fibrillation in the more populated European countries: perspectives from the GARFIELD-AF registry. Eur Heart J 2017;38:4603.
- Badhwar V, Rankin JS, Ad N, et al. Surgical Ablation of Atrial Fibrillation in the United States: Trends and Propensity Matched Outcomes. Ann Thorac Surg 2017;104:493-500. [Crossref] [PubMed]
- Churyla A, Andrei AC, Kruse J, et al. Safety of Atrial Fibrillation Ablation With Isolated Surgical Aortic Valve Replacement. Ann Thorac Surg 2021;111:809-17. [Crossref] [PubMed]
- Gammie JS, Haddad M, Milford-Beland S, et al. Atrial fibrillation correction surgery: lessons from the Society of Thoracic Surgeons National Cardiac Database. Ann Thorac Surg 2008;85:909-14. [Crossref] [PubMed]
- Malaisrie SC, McCarthy PM, Kruse J, et al. Burden of preoperative atrial fibrillation in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 2018;155:2358-2367.e1. [Crossref] [PubMed]
- McCarthy PM, Davidson CJ, Kruse J, et al. Prevalence of atrial fibrillation before cardiac surgery and factors associated with concomitant ablation. J Thorac Cardiovasc Surg 2020;159:2245-2253.e15. [Crossref] [PubMed]
- Darby AE, Dimarco JP. Management of atrial fibrillation in patients with structural heart disease. Circulation 2012;125:945-57. [Crossref] [PubMed]
- Zhou X, Otsuji Y, Yoshifuku S, et al. Impact of atrial fibrillation on tricuspid and mitral annular dilatation and valvular regurgitation. Circ J 2002;66:913-6. [Crossref] [PubMed]
- Qin D, Mansour MC, Ruskin JN, et al. Atrial Fibrillation-Mediated Cardiomyopathy. Circ Arrhythm Electrophysiol 2019;12:e007809. [Crossref] [PubMed]
- Attaran S, Shaw M, Bond L, et al. A comparison of outcome in patients with preoperative atrial fibrillation and patients in sinus rhythm. Ann Thorac Surg 2011;92:1391-5. [Crossref] [PubMed]
- Ngaage DL, Schaff HV, Mullany CJ, et al. Influence of preoperative atrial fibrillation on late results of mitral repair: is concomitant ablation justified? Ann Thorac Surg 2007;84:434-42; discussion 442-3. [Crossref] [PubMed]
- Ngaage DL, Schaff HV, Mullany CJ, et al. Does preoperative atrial fibrillation influence early and late outcomes of coronary artery bypass grafting? J Thorac Cardiovasc Surg 2007;133:182-9. [Crossref] [PubMed]
- Quader MA, McCarthy PM, Gillinov AM, et al. Does preoperative atrial fibrillation reduce survival after coronary artery bypass grafting? Ann Thorac Surg 2004;77:1514-22; discussion 1522-4. [Crossref] [PubMed]
- Biviano AB, Nazif T, Dizon J, et al. Atrial Fibrillation Is Associated With Increased Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement: Insights From the Placement of Aortic Transcatheter Valve (PARTNER) Trial. Circ Cardiovasc Interv 2016;9:e002766. [Crossref] [PubMed]
- Brener MI, George I, Kosmidou I, et al. Atrial Fibrillation Is Associated With Mortality in Intermediate Surgical Risk Patients With Severe Aortic Stenosis: Analyses From the PARTNER 2A and PARTNER S3i Trials. J Am Heart Assoc 2021;10:e019584. [Crossref] [PubMed]
- Keßler M, Pott A, Mammadova E, et al. Atrial Fibrillation Predicts Long-Term Outcome after Transcatheter Edge-to-Edge Mitral Valve Repair by MitraClip Implantation. Biomolecules 2018;8:152. [Crossref] [PubMed]
- Sun F, Liu H, Zhang Q, et al. Impact of atrial fibrillation on outcomes of patients treated by transcatheter mitral valve repair: A systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e22195. [Crossref] [PubMed]
- Shah S, Raj V, Abdelghany M, et al. Impact of atrial fibrillation on the outcomes of transcatheter mitral valve repair using MitraClip: a systematic review and meta-analysis. Heart Fail Rev 2021;26:531-43. [Crossref] [PubMed]
- Badhwar V, Rankin JS, Damiano RJ Jr, et al. The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann Thorac Surg 2017;103:329-41. [Crossref] [PubMed]
- Ad N, Damiano RJ Jr, Badhwar V, et al. Expert consensus guidelines: Examining surgical ablation for atrial fibrillation. J Thorac Cardiovasc Surg 2017;153:1330-1354.e1. [Crossref] [PubMed]
- Cox JL, Schuessler RB, D'Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991;101:569-83.
- Gaynor SL, Diodato MD, Prasad SM, et al. A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg 2004;128:535-42. [Crossref] [PubMed]
- Philpott JM, Zemlin CW, Cox JL, et al. The ABLATE Trial: Safety and Efficacy of Cox Maze-IV Using a Bipolar Radiofrequency Ablation System. Ann Thorac Surg 2015;100:1541-6; discussion 1547-8. [Crossref] [PubMed]
- Schill MR, Melby SJ, Speltz M, et al. Evaluation of a Novel Cryoprobe for Atrial Ablation in a Chronic Ovine Model. Ann Thorac Surg 2017;104:1069-73. [Crossref] [PubMed]
- Weimar T, Lee AM, Ray S, et al. Evaluation of a novel cryoablation system: in vivo testing in a chronic porcine model. Innovations (Phila) 2012;7:410-6. [Crossref] [PubMed]
- Cox JL. The Minimally Invasive Maze-III Procedure. Operat Techn Cardiovasc Thorac Surg 2000;5:79-92.
- Vural Ü, Balcı AY, Ağlar AA, et al. Which Method to Use for Surgical Ablation of Atrial Fibrillation Performed Concomitantly with Mitral Valve Surgery: Radiofrequency Ablation versus Cryoablation. Braz J Cardiovasc Surg 2018;33:542-52. [Crossref] [PubMed]
- Ad N, Holmes SD, Rongione AJ, et al. Does Surgical Ablation Energy Source Affect Long-Term Success of the Concomitant Cox Maze Procedure? Ann Thorac Surg 2017;104:29-35. [Crossref] [PubMed]
- Gillinov AM, Gelijns AC, Parides MK, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med 2015;372:1399-409. [Crossref] [PubMed]
- McCarthy PM, Gerdisch M, Philpott J, et al. Three-year outcomes of the postapproval study of the AtriCure Bipolar Radiofrequency Ablation of Permanent Atrial Fibrillation Trial. J Thorac Cardiovasc Surg 2022;164:519-527.e4. [Crossref] [PubMed]
- Khiabani AJ, MacGregor RM, Bakir NH, et al. The long-term outcomes and durability of the Cox-Maze IV procedure for atrial fibrillation. J Thorac Cardiovasc Surg 2022;163:629-641.e7. [Crossref] [PubMed]
- Ad N, Holmes SD, Massimiano PS, et al. Long-term outcome following concomitant mitral valve surgery and Cox maze procedure for atrial fibrillation. J Thorac Cardiovasc Surg 2018;155:983-94. [Crossref] [PubMed]
- Musharbash FN, Schill MR, Sinn LA, et al. Performance of the Cox-maze IV procedure is associated with improved long-term survival in patients with atrial fibrillation undergoing cardiac surgery. J Thorac Cardiovasc Surg 2018;155:159-70. [Crossref] [PubMed]
- Suwalski P, Kowalewski M, Jasiński M, et al. Survival after surgical ablation for atrial fibrillation in mitral valve surgery: Analysis from the Polish National Registry of Cardiac Surgery Procedures (KROK). J Thorac Cardiovasc Surg 2019;157:1007-1018.e4. [Crossref] [PubMed]
- Suwalski P, Kowalewski M, Jasiński M, et al. Surgical ablation for atrial fibrillation during isolated coronary artery bypass surgery. Eur J Cardiothorac Surg 2020;57:691-700. [Crossref] [PubMed]
- Kowalewski M, Jasiński M, Staromłyński J, et al. Long-Term Survival Following Surgical Ablation for Atrial Fibrillation Concomitant to Isolated and Combined Coronary Artery Bypass Surgery-Analysis from the Polish National Registry of Cardiac Surgery Procedures (KROK). J Clin Med 2020;9:1345. [Crossref] [PubMed]
- Iribarne A, DiScipio AW, McCullough JN, et al. Surgical Atrial Fibrillation Ablation Improves Long-Term Survival: A Multicenter Analysis. Ann Thorac Surg 2019;107:135-42. [Crossref] [PubMed]
- Okum E. Non Atriotomy Epicardial Ablation for AF. CTSNet [Internet]. 2021.
- Weimar T, Gaynor SL, Seubert DY, et al. Performing the Left Atrial Maze Ablation Pattern Without Atriotomy. Ann Thorac Surg 2016;101:777-9. [Crossref] [PubMed]
- Ad N, Holmes SD, Rongione AJ, et al. The long-term safety and efficacy of concomitant Cox maze procedures for atrial fibrillation in patients without mitral valve disease. J Thorac Cardiovasc Surg 2019;157:1505-14. [Crossref] [PubMed]
- Lee AM, Clark K, Bailey MS, et al. A minimally invasive cox-maze procedure: operative technique and results. Innovations (Phila) 2010;5:281-6. [Crossref] [PubMed]
- Lawrance CP, Henn MC, Miller JR, et al. A minimally invasive Cox maze IV procedure is as effective as sternotomy while decreasing major morbidity and hospital stay. J Thorac Cardiovasc Surg 2014;148:955-61; discussion 962-2. [Crossref] [PubMed]
- Massimiano PS, Yanagawa B, Henry L, et al. Minimally invasive fibrillating heart surgery: a safe and effective approach for mitral valve and surgical ablation for atrial fibrillation. Ann Thorac Surg 2013;96:520-7. [Crossref] [PubMed]
- Roberts HG, Wei LM, Dhamija A, et al. Robotic assisted cryothermic biatrial Cox-Maze. J Cardiovasc Electrophysiol 2021;32:2879-83. [Crossref] [PubMed]
- Badhwar V. Robotic-assisted biatrial Cox-maze ablation for atrial fibrillation. J Thorac Cardiovasc Surg 2023;165:108-12. [Crossref] [PubMed]
- Goebel N, Brandel-Ursulescu C, Tanriverdi S, et al. Partial upper sternotomy for concomitant left atrial ablation and aortic valve replacement. J Cardiovasc Surg (Torino) 2021;62:87-94. [Crossref] [PubMed]
- Kolakowski S Jr, Woo YJ. Minimally invasive aortic valve replacement combined with radiofrequency-modified maze procedure. J Card Surg 2005;20:164-6. [Crossref] [PubMed]
- Totsugawa T, Hiraoka A, Tamura K, et al. Clamp Ablation of Pulmonary Veins During Minimally Invasive Aortic Valve Replacement. Ann Thorac Surg 2017;104:e471-3. [Crossref] [PubMed]
- Cox JL, Ad N. The importance of cryoablation of the coronary sinus during the Maze procedure. Semin Thorac Cardiovasc Surg 2000;12:20-4. [Crossref] [PubMed]
- Cox JL, Malaisrie SC, Churyla A, et al. Cryosurgery for Atrial Fibrillation: Physiologic Basis for Creating Optimal Cryolesions. Ann Thorac Surg 2021;112:354-62. [Crossref] [PubMed]
- Khiabani AJ, MacGregor RM, Manghelli JL, et al. Bipolar Radiofrequency Ablation on Explanted Human Hearts: How to Ensure Transmural Lesions. Ann Thorac Surg 2020;110:1933-9. [Crossref] [PubMed]
- McCarthy PM. The maze IV operation is not always the best choice: Matching the procedure to the patient. JTCVS Techniques 2021. doi:
10.1016/j.xjtc.2021.06.031 . - Cox JL, Ad N, Churyla A, et al. The Maze Procedure and Postoperative Pacemakers. Ann Thorac Surg 2018;106:1561-9. [Crossref] [PubMed]
- Lim HS, Hocini M, Dubois R, et al. Complexity and Distribution of Drivers in Relation to Duration of Persistent Atrial Fibrillation. J Am Coll Cardiol 2017;69:1257-69. [Crossref] [PubMed]
- Cochet H, Mouries A, Nivet H, et al. Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed-enhanced magnetic resonance imaging in a general cardiology population. J Cardiovasc Electrophysiol 2015;26:484-92. [Crossref] [PubMed]
- Voeller RK, Bailey MS, Zierer A, et al. Isolating the entire posterior left atrium improves surgical outcomes after the Cox maze procedure. J Thorac Cardiovasc Surg 2008;135:870-7. [Crossref] [PubMed]
- Henn MC, Lancaster TS, Miller JR, et al. Late outcomes after the Cox maze IV procedure for atrial fibrillation. J Thorac Cardiovasc Surg 2015;150:1168-76, 1178.e1-2.
- DeLurgio DB, Crossen KJ, Gill J, et al. Hybrid Convergent Procedure for the Treatment of Persistent and Long-Standing Persistent Atrial Fibrillation: Results of CONVERGE Clinical Trial. Circ Arrhythm Electrophysiol 2020;13:e009288. [Crossref] [PubMed]
- Rivner H, Mitrani RD, Goldberger JJ. Atrial Myopathy Underlying Atrial Fibrillation. Arrhythm Electrophysiol Rev 2020;9:61-70. [Crossref] [PubMed]
- Di Biase L, Burkhardt JD, Mohanty P, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation 2010;122:109-18. [Crossref] [PubMed]
- Cox JL, Ad N, Palazzo T. Impact of the maze procedure on the stroke rate in patients with atrial fibrillation. J Thorac Cardiovasc Surg 1999;118:833-40. [Crossref] [PubMed]
- Whitlock RP, Belley-Cote EP, Paparella D, et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N Engl J Med 2021;384:2081-91. [Crossref] [PubMed]
- Healey JS, Crystal E, Lamy A, et al. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J 2005;150:288-93. [Crossref] [PubMed]
- Kanderian AS, Gillinov AM, Pettersson GB, et al. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol 2008;52:924-9. [Crossref] [PubMed]
- Katz ES, Tsiamtsiouris T, Applebaum RM, et al. Surgical left atrial appendage ligation is frequently incomplete: a transesophageal echocardiograhic study. J Am Coll Cardiol 2000;36:468-71. [Crossref] [PubMed]
- García-Fernández MA, Pérez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol 2003;42:1253-8. [Crossref] [PubMed]
- Aryana A, Singh SK, Singh SM, et al. Association between incomplete surgical ligation of left atrial appendage and stroke and systemic embolization. Heart Rhythm 2015;12:1431-7. [Crossref] [PubMed]
- Güner A, Kalçık M, Gündüz S, et al. The relationship between incomplete surgical obliteration of the left atrial appendage and thromboembolic events after mitral valve surgery (from the ISOLATE Registry). J Thromb Thrombolysis 2021;51:1078-89. [Crossref] [PubMed]
- Cullen MW, Stulak JM, Li Z, et al. Left Atrial Appendage Patency at Cardioversion After Surgical Left Atrial Appendage Intervention. Ann Thorac Surg 2016;101:675-81. [Crossref] [PubMed]
- Ailawadi G, Gerdisch MW, Harvey RL, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg 2011;142:1002-9, 1009.e1.
- Emmert MY, Puippe G, Baumüller S, et al. Safe, effective and durable epicardial left atrial appendage clip occlusion in patients with atrial fibrillation undergoing cardiac surgery: first long-term results from a prospective device trial. Eur J Cardiothorac Surg 2014;45:126-31. [Crossref] [PubMed]
- Caliskan E, Eberhard M, Falk V, et al. Incidence and characteristics of left atrial appendage stumps after device-enabled epicardial closure. Interact Cardiovasc Thorac Surg 2019;29:663-9. [Crossref] [PubMed]
- Ad N, Massimiano PS, Shuman DJ, et al. New Approach to Exclude the Left Atrial Appendage During Minimally Invasive Cryothermic Surgical Ablation. Innovations (Phila) 2015;10:323-7. [Crossref] [PubMed]
- Rhee Y, Park SJ, Lee JW. Epicardial left atrial appendage clip occlusion in patients with atrial fibrillation during minimally invasive cardiac surgery. J Thorac Cardiovasc Surg 2021; Epub ahead of print. [Crossref]
- Damiano RJ Jr, Schwartz FH, Bailey MS, et al. The Cox maze IV procedure: predictors of late recurrence. J Thorac Cardiovasc Surg 2011;141:113-21. [Crossref] [PubMed]
- Ad N, Henry L, Hunt S, et al. The implementation of a comprehensive clinical protocol improves long-term success after surgical treatment of atrial fibrillation. J Thorac Cardiovasc Surg 2010;139:1146-52. [Crossref] [PubMed]
Cite this article as: Gerdisch MW. The most beneficial options for patients with arrhythmia and concomitant structural heart disease: a review. AME Surg J 2023;3:23.


