
Idiopathic granulomatous mastitis in pregnancy: a case report on a new management approach using azathioprine and allopurinol
Introduction
Idiopathic granulomatous mastitis (IGM) is a rare, benign inflammatory disease of the breast (1). It is characterised histologically by non-caseating lobulocentric granulomatous inflammation and usually affects one side only (2). It is commonly seen in women of child-bearing age and is known to have a strong association with pregnancy and lactation although the exact aetiology is unknown (2,3). The clinical presentation includes a palpable breast mass with inflammation, skin ulceration, sinus and fistula formation with discharge. Diagnosis can be challenging because the condition is often mistaken for infection and a period of ineffective antibiotic therapy can delay correct treatment. Other diagnoses such as inflammatory breast cancer and systemic inflammatory conditions will need to be ruled out before a formal diagnosis of IGM is established. The Corynebacterium kroppenstedtii bacterium is widely associated with the onset of IGM and can be tested for on bacterial cultures. In diagnosing IGM, histological features should include granulomatous inflammation.
There are currently no consensus guidelines for treating IGM and there are few cases published on its management during pregnancy. The most recently published case reports favour oral glucocorticoids and methotrexate as first line treatments but chronic use of both drugs is typically contraindicated in pregnancy and breast-feeding (1,2,4-7). Given the complexities of both diagnosis and management, IGM should ideally be managed via a multi-disciplinary approach (8).
We report a case of IGM in a pregnant woman successfully treated using a new regimen of azathioprine and allopurinol. This has guided the development of a new complex breast abscess pathway involving breast surgeons, gastroenterologists, infectious diseases specialists, radiologists and pathologists to diagnose and manage IGM. We present the following case in accordance with the CARE reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-22-8/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 31-year-old Caucasian female, four months post-partum and actively breast feeding, presented with a 4-week history of a tender left breast mass with overlying skin discolouration. She was previously fit and well, and was a non-smoker with no past medical, drug or family history. She was treated empirically with two consecutive courses of antibiotics (flucloxacillin followed by co-amoxiclav) for presumed lactational mastitis by the general practitioner (GP). Urgent two week wait referral to the breast unit was made on worsening of symptoms and development of a painful fluctuant breast mass which required ultrasound guided drainage. An inflammatory breast cancer was ruled out and the pus culture sample had no growth of bacterial organisms. Co-amoxiclav was continued.
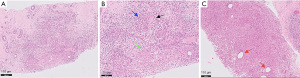
Over the following months her symptoms progressed with features of new ulceration and large volume pustular discharge. She also developed a second inflammatory mass with additional smaller collections and the formation several sinuses. Repeat ultrasound scans were performed with pus aspirations for microscopy and culture (Figure 1) which grew Staphylococcus Aureus with sensitivity to clindamycin. She was therefore started on clindamycin for 2 weeks. Over the next 6 weeks she continued to develop multiple new areas of ulceration with discharging sinuses. New pus cultures grew Acinetobacter ursingii and Enterobacter faecalis. Due to the protracted course of the disease at 12 weeks following her initial presentation, the first core biopsy of the breast tissue was performed. This showed lobulocentric inflammation, non-caseating granulomas and microabscesses (Figure 2) (9). The mixed inflammatory infiltrate composed of lymphocytes, plasma cells, neutrophils. Special stains for fungal elements (Periodic acid-Shiff diastase) and mycobacteria (Ziehl-Neelsen) were negative. Recommendation from the Breast MDT was to opt for sensitivity-targeted antibiotic therapy management and termination of lactation using cabergoline.
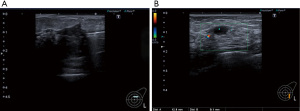
At four months from initial presentation with no symptomatic improvement, a diagnosis of presumed IGM was made. Referral to the Infectious Diseases Unit to rule systemic inflammatory diseases and other infective granulomatous conditions (tuberculosis or complex non-tuberculous mycobacterial infection) was done. Fresh large bore core biopsy samples were taken for prolonged cultures and showed no evidence of mycobacterium infection. Antibiotic therapy was then stopped and daily oral prednisolone was commenced at 30 mg once daily for four weeks. Almost immediate clinical improvement was seen but it was a challenge in balancing the side effects of the steroids (diarrhoea, abdominal discomfort, weight gain) with the quiescence of symptoms. A reducing dose regimen was commenced with a reduction of prednisolone by 5 mg every week. Unfortunately, her symptoms worsened once the dose of prednisolone went below 10 mg daily and re-initiation of 30 mg prednisolone was required to regain control. Re-evaluation of the most appropriate immunosuppressive regimen was necessary after concerns were raised with steroid dependence, steroid side effects and the planning of a second pregnancy.
Azathioprine has a lower long term side effect profile compared to glucocorticoids and is considered appropriate as a maintenance immunosuppressant (10). It is low risk in pregnancy when deemed clinically necessary unlike methotrexate which is absolutely contraindicated (11). Gastroenterology input was required due to their familiarity of azathioprine and expertise in ruling out IGM as an extra-intestinal manifestation of inflammatory bowel disease (IBD) (normal calprotectin and colonoscopy) and granulomatous hepatitis (normal liver ultrasound scan and serum liver function tests). Given the patient’s sub-therapeutic 6-methylmercaptopurine levels on pre-monitoring blood tests, allopurinol 100 mg was given in combination with a lower dose of azathioprine 50 mg. Whilst this combination of drugs has a recognised interaction risk of haematological toxicity, once carefully monitored can have a desired anti-inflammatory effect and low side effect profile (12-14). An initial short course of tapering 30 mg Prednisolone was re-started whilst azathioprine was titrated into therapeutic range. Response to this new drug regimen showed immediate and significant improvement of inflammation, tenderness and no further discharge from sinuses. Prior to initiation of this regimen, full safety checking to limit side effects to azathioprine was done (Table 1) (15).
Table 1
| Thiopurine methyltransferase levels |
| Hepatitis B and C serology |
| Human immunodeficiency virus |
| Varicella zoster exposure testing |
| Chest X-ray (to exclude to previous tuberculosis) |
| Full blood count |
| Renal function tests |
| Liver function tests |
| QuantiFERON-TB gold |
| Strongyloides serology |
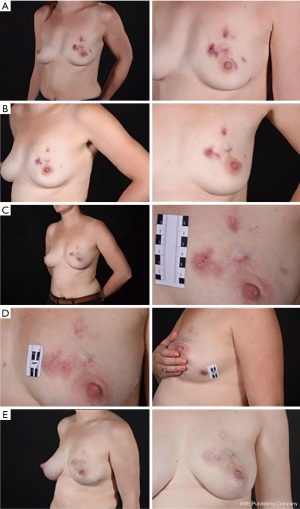
A year following initiation of treatment the patient had a successful second pregnancy whilst remaining on the same regimen. She reported one minor flare at 19 weeks into pregnancy which was due to reduced compliance with medication and was quickly controlled once recommenced. Otherwise, her disease remained quiescent throughout the pregnancy and into the post-partum period. She had an uncomplicated labour and delivered a healthy baby. Currently at 8 months post-partum, the patient has had no recurrence or relapse of IGM and remains on the same medication with no side effects. She is under surveillance once every four months by the breast and gastroenterology teams. Figure 3 shows photographic documentation of the clinical course and a detailed timeline can be found in Table S1.
Discussion
IGM is a rare condition with an incidence of 2.4/100,000 (3). Due to the low incidence, published data for this condition exists mainly in the form of retrospective studies and small case series. There is a lack of consensus for optimal treatment which has made this condition challenging for clinicians to manage. The management of IGM using azathioprine in a cross discipline approach has not previously been reported in pregnancy and lactation. Current described treatment regimens include antibiotic therapy, oral corticosteroids, immunosuppressive therapies and surgical intervention (3). Surgical management can range from incision and drainage, excisional biopsy, partial or complete mastectomy to immediate or delayed breast reconstruction (16). A systematic review and meta-analysis performed by Ma et al. showed that surgical management was the best and fastest way to complete eradication of IGM for patients not concerned with surgical scarring (17). Breast conserving options such as wide local excision have been associated with a high recurrence rate, delayed wound healing, fistula formation, extensive scarring and poor cosmesis (18). A percentage of these patients will then require concurrent steroid therapy. The option of surgical management is also limited in pregnancy.
Early presentation mimics infective conditions such as mastitis and breast abscess and diagnosis is therefore often delayed while empirical antibiotic therapy is commenced. Antibiotics have shown relatively low success rates (6–21% clinical improvement) as IGM is classically a non-infective inflammatory condition; however, there are cases with superimposed infection, as demonstrated in this patient, in which they can be beneficial (3). While needle aspiration for microbiology is common practice to rule out infection, taking core biopsies is not. However, histological assessment is usually performed after antimicrobial treatment fails and it becomes necessary to consider alternative diagnoses, including inflammatory breast cancer.
The key histological feature of IGM is granulomatous inflammation centred on the breast lobule (9,19). This spatial relationship with the lobule is less conspicuous in other causes of granulomatous inflammation that may enter the differential diagnosis, such as mammary duct ectasia and sarcoidosis, although the lobule may eventually become destroyed and replaced by sheets of inflammatory cells (9,19,20). In addition to the granulomatous/histiocytic component, which includes Langhans-type giant cells, variable numbers of lymphocytes, plasma cells, eosinophils and neutrophil polymorphs are seen, the latter of which may form microabscesses. Clear spaces thought to represent lipid are sometimes noted within the inflammation or granulomata and histiocytes with cytoplasmic accumulation of lipid may develop a foamy appearance (9). Other features include fat necrosis and ductal inflammation (9,19). It has been suggested that ductal damage, due to either inflammation or trauma could be the precipitating event in IGM, with the resultant leakage of secretory material into the stroma initiating the granulomatous response (20).
The Corynebacterium species of bacteria is widely detected in granulomatous mastitis but the relationship between them is not yet definitive. Literature indicates a potential causal relationship between cystic neutrophilic granulomatous mastitis (CNGM) and Corynebacterium, with Corynebacterium kroppenstedtii being the most commonly occurring organism of the species (21). Patients with Corynebacterium infections are more likely to present with fever and sinus formation. In such cases, the pathologist should investigate for any primary bacterial infection and consider culture of fresh specimens, gram staining or rRNA gene sequencing of specimens obtained at surgery (22). Early recognition of CNGM may warrant first line treatment with lipophilic antimicrobials such as clindamycin (21).
The diagnosis of IGM is made once all other causes (including all infective, other granulomatous conditions and neoplasm) have been excluded (Table 2) (23). Once a diagnosis of IGM is made, most clinicians prescribe short courses of oral glucocorticoids as first-line treatment. Patients generally respond well to glucocorticoids with reported symptom resolution between 66–72% (3). However, once the dose is tapered, the rate of recurrence can be as high as 20% (24). Long courses of corticosteroids are not recommended due to the extensive side effect profile in a relatively young patient cohort. Important side effects include osteoporosis, diabetes mellitus, peptic ulcer disease, significant weight gain, insomnia, avascular necrosis and predisposition to infection. Its use is avoided in pregnancy due to the increased risk of teratogenesis, gestational diabetes, preterm delivery, interaction with obstetric medication (specifically mifepristone) and concerns with hypothalamic-pituitary-adrenal (HPA) axis alteration in neonates (25).
Table 2
| Neoplastic |
| Inflammatory carcinoma of the breast |
| Inflammatory |
| Idiopathic inflammatory mastitis |
| Infectious |
| Breast abscess (bacterial or mycobacterial) |
| Tuberculous mastitis |
| Histoplasmosis |
| Autoimmune |
| Sarcoidosis |
| Granulomatosis with polyangiitis (formerly Wegener’s granulomatosis) |
| Inflammatory bowel disease |
| Granulomatous hepatitis |
| Systemic lupus erythematosus |
| Common variable immunodeficiency |
| Rheumatoid arthritis |
| Anti-neutrophil cytoplastic autoantibody vasculitis |
The use of immunosuppressants, particularly methotrexate, as second-line treatment has also been reported in the literature (26). The use of methotrexate in combination with corticosteroids has shown promising results however the efficacy of methotrexate as monotherapy is still largely unknown with only two recently published studies available (27-29). Moreover, the side effect profile of the drug and its absolute contraindication in both pregnancy and breast-feeding render it an unsuitable option for the majority of the IGM patient cohort.
Azathioprine, a thiopurine metabolite, is an alternative immunosuppressant with fewer side effects and can be administered in pregnancy and breast-feeding when clinically deemed necessary (10,11). To date, there are only 5 publications (1 case report and 4 small case series) describing the treatment IGM using azathioprine in combination with glucocorticoids, with no studies describing azathioprine as monotherapy (30-35).
Safe prescription of azathioprine requires pre-treatment drug counselling and attentive therapeutic monitoring (36). Thiopurine methyltransferase (TPMT) levels can be measured to determine a patient’s level of risk to myelosuppression before starting treatment and 6-Thioguanine nucleotide (6-TGN) levels to check a therapeutic level of the active metabolite has been achieved. Patients are co-prescribed allopurinol when this has not been achievable and their 6-TGN levels are subtherapeutic, or when they are suspected to have side-effects to high levels of methyl-mercaptopurine. A low dose of allopurinol 100 mg daily is sufficient to induce the noted metabolic switch in this cohort of patients (37). The dose of azathioprine should be reduced by 25% of the recommended dose and guided with a careful monitoring regimen with regular blood tests (full blood count and liver function tests). Allopurinol acts to inhibit the second step of metabolism of azathioprine to 6-mercaptopurine and can result in higher levels of this which can result in haematological toxicity (38). The white cell count (WCC) should be checked every week for the first 4 weeks after allopurinol is added, then once every fortnight for the next 4 weeks and then once every three months long term. This is to monitor for blood dyscrasias, leukopenia, thrombocytopenia or pancytopenia (38). Metabolite levels should also be checked approximately 4 weeks after starting allopurinol to ensure therapeutic levels have been attained and to allow for further azathioprine dose adjustment if required (37). Increasingly in clinical practice, some patients request an immunosuppressive regimen associated with the lowest probability of side-effects, and this approach of low dose azathioprine with allopurinol can be considered if suitable. This has been shown to be effective and is more tolerable (12-15).
As azathioprine is a drug that is not routinely used by breast surgeons, joint care with another specialty with this expertise is recommended. A small number of publications on IGM management have described adopting such a multidisciplinary approach and usually include collaboration with Rheumatology physicians (38). In this case, the existing expertise that gastroenterologists have using azathioprine to treat patients with IBD led to the establishment of a joint pathway for this patient. There are some similar histological characteristics, including the presence of non-caseating granulomas, shared between IGM and IBD. Reports of patients with both IGM and IBD have described improvements in both conditions when treated with azathioprine (38).
This case highlights the challenges of diagnosing and treating IGM. As with many patients with IGM, there was a prolonged period of suboptimal management with unnecessary extended antibiotic and glucocorticoid therapy. The patient’s young age and wish for a second pregnancy was a driving factor to consider other approaches to controlling her disease. Our experience in this case has led to the development of a multidisciplinary approach towards the management of complex breast abscesses involving breast surgeons, radiologists, pathologists, infective diseases specialists and gastroenterologists. This treatment algorithm (Figure 4) aims to reduce diagnostic delays and to expedite optimum treatment for all patients with IGM including those who are pregnant or lactating.
The possibility of a diagnosis of IGM should be considered in all inflammatory breast masses that have an atypical presentation (large solid component, sinus formation, fistulation, multiple areas) or after a short period of failed empirical antibiotic therapy (1–2 weeks). Core biopsy at this early stage is recommended for the exclusion of neoplasm, to demonstrate the presence of granulomas and to provide good quality tissue for prolonged microbiology cultures including acid fast bacilli. We recommend that the identification of granulomas on core biopsy leads directly to referral to the infectious diseases team who can perform chest radiographs, QuantiFERON testing, test for sarcoidosis and review of culture and antimicrobial therapy. This stage is important as immunosuppressive therapy for IGM can only be commenced once non-caseating granulomas have been demonstrated and all infective causes have been excluded. This stage, even when expedited could take several weeks. Once the diagnosis of IGM is made, the breast team can safely attempt a single course of oral glucocorticoid therapy. High dose prednisolone can be maintained for 4 weeks once symptoms have resolved and then tapered with a reducing regimen. To avoid long term sequelae from steroid therapy, the aim is to stop steroids completely after the tapering regimen and not to maintain disease control on a low dose of steroid as is the approach with other inflammatory conditions. Disease relapse within 1-year after a single course of steroids, or a relapse while weaning the initial course of steroids should trigger referral a to a specialist with expertise in immunosuppressive therapy to discuss the benefits of steroid sparing treatments such as azathioprine or methotrexate. This could be the rheumatologists or gastroenterologists.
This case demonstrated that long term disease control can be effectively maintained using single immunosuppressive therapy with azathioprine even in pregnancy and whilst breastfeeding. A multidisciplinary approach was important to safely achieve this outcome. She will undergo planned interval azathioprine withdrawal in the future while under joint care of the breast surgeons and gastroenterologists.
Learning points to guide clinicians on the management of IGM can be found in Table 3 and the patient perspective can be found in Appendix 1.
Table 3
| IGM is a rare condition that is difficult to treat due to limited data and lack of treatment consensus |
| Early core biopsies for microbiology and histology should be performed in inflammatory breast masses with atypical appearances (large solid component, sinus formation or fistulation) or after a short period of failed antimicrobial therapy (1–2 weeks) |
| A multidisciplinary pathway involving breast surgeons, radiologists, pathologists, infectious diseases specialists and a specialty with expertise in prescribing azathioprine (rheumatology or gastroenterology) is recommended |
| Azathioprine is effective in IGM including in women who are pregnant and/or breast-feeding |
| Azathioprine monotherapy avoids the detrimental side effects of long term glucocorticoid use |
IGM, idiopathic granulomatous mastitis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-22-8/rc
Peer Review File: Available at https://asj.amegroups.com/article/view/10.21037/asj-22-8/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-22-8/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol 1972;58:642-6. [Crossref] [PubMed]
- Pluguez-Turull CW, Nanyes JE, Quintero CJ, et al. Idiopathic Granulomatous Mastitis: Manifestations at Multimodality Imaging and Pitfalls. Radiographics 2018;38:330-56. [Crossref] [PubMed]
- Wolfrum A, Kümmel S, Theuerkauf I, et al. Granulomatous Mastitis: A Therapeutic and Diagnostic Challenge. Breast Care (Basel) 2018;13:413-8. [Crossref] [PubMed]
- Hashmi D, Al Samaraee A, Marks B, et al. Idiopathic granulomatous mastitis: a diagnostic dilemma. Br J Hosp Med (Lond) 2020;81:1-4. [Crossref] [PubMed]
- Brennan ME, Morgan M, Heilat GB, et al. Granulomatous lobular mastitis: Clinical update and case study. Aust J Gen Pract 2020;49:44-7. [Crossref] [PubMed]
- National Institute for Health and Care Excellence. METHOTREXATE | Drug | BNF content published by NICE [Internet]. Bnf.nice.org.uk. [cited 17 March 2020]. Available online: https://bnf.nice.org.uk/drug/methotrexate.html#pregnancy
- National Institute for Health and Care Excellence. PREDNISOLONE | Drug | BNF content published by NICE [Internet]. Bnf.nice.org.uk. 2020 [cited 17 March 2020]. Available online: https://bnf.nice.org.uk/drug/prednisolone.html#pregnancy
- Yaghan RJ, Ayoub NM, Hamouri S, et al. The Role of Establishing a Multidisciplinary Team for Idiopathic Granulomatous Mastitis in Improving Patient Outcomes and Spreading Awareness about Recent Disease Trends. Int J Breast Cancer 2020;2020:5243958. [Crossref] [PubMed]
- Going JJ, Anderson TJ, Wilkinson S, et al. Granulomatous lobular mastitis. J Clin Pathol 1987;40:535-40. [Crossref] [PubMed]
- van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis 2015;9:107-24. [Crossref] [PubMed]
- Hutson JR, Matlow JN, Moretti ME, et al. The fetal safety of thiopurines for the treatment of inflammatory bowel disease in pregnancy. J Obstet Gynaecol 2013;33:1-8. [Crossref] [PubMed]
- Friedman AB, Brown SJ, Bampton P, et al. Randomised clinical trial: efficacy, safety and dosage of adjunctive allopurinol in azathioprine/mercaptopurine nonresponders (AAA Study). Aliment Pharmacol Ther 2018;47:1092-102. [Crossref] [PubMed]
- Kiszka-Kanowitz M, Theede K, Mertz-Nielsen A. Randomized clinical trial: a pilot study comparing efficacy of low-dose azathioprine and allopurinol to azathioprine on clinical outcomes in inflammatory bowel disease. Scand J Gastroenterol 2016;51:1470-5. [Crossref] [PubMed]
- Hoentjen F, Seinen ML, Hanauer SB, et al. Safety and effectiveness of long-term allopurinol-thiopurine maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis 2013;19:363-9. [Crossref] [PubMed]
- Goel RM, Blaker P, Mentzer A, et al. Optimizing the use of thiopurines in inflammatory bowel disease. Ther Adv Chronic Dis 2015;6:138-46. [Crossref] [PubMed]
- Yau FM, Macadam SA, Kuusk U, et al. The surgical management of granulomatous mastitis. Ann Plast Surg 2010;64:9-16. [Crossref] [PubMed]
- Ma X, Min X, Yao C. Different Treatments for Granulomatous Lobular Mastitis: A Systematic Review and Meta-Analysis. Breast Care (Basel) 2020;15:60-6. [Crossref] [PubMed]
- Shin YD, Park SS, Song YJ, et al. Is surgical excision necessary for the treatment of Granulomatous lobular mastitis? BMC Womens Health 2017;17:49. [Crossref] [PubMed]
- Fletcher A, Magrath IM, Riddell RH, et al. Granulomatous mastitis: a report of seven cases. J Clin Pathol 1982;35:941-5. [Crossref] [PubMed]
- Davies JD, Burton PA. Postpartum lobular granulomatous mastitis. J Clin Pathol 1983;36:363. [Crossref] [PubMed]
- Wang Y, LeGolvan M, Chapin K, et al. Cystic neutrophilic granulomatous mastitis with corynebacterium and staphylococcus mimicking breast carcinoma. Clin Case Rep 2018;6:2208-10. [Crossref] [PubMed]
- Johnstone KJ, Robson J, Cherian SG, et al. Cystic neutrophilic granulomatous mastitis associated with Corynebacterium including Corynebacterium kroppenstedtii. Pathology 2017;49:405-12. [Crossref] [PubMed]
- Sripathi S, Ayachit A, Bala A, et al. Idiopathic granulomatous mastitis: a diagnostic dilemma for the breast radiologist. Insights Imaging 2016;7:523-9. [Crossref] [PubMed]
- Lei X, Chen K, Zhu L, et al. Treatments for Idiopathic Granulomatous Mastitis: Systematic Review and Meta-Analysis. Breastfeed Med 2017;12:415-21. [Crossref] [PubMed]
- AlSaad D, Lindow S, Lee BH, et al. Maternal, fetal, and neonatal outcomes associated with long-term use of corticosteroids during pregnancy. Obstet Gynaeco 2019;21:117-25. [Crossref]
- Schmajuk G, Genovese MC. First report of idiopathic granulomatous mastitis treated with methotrexate monotherapy. J Rheumatol 2009;36:1559-60. [Crossref] [PubMed]
- Aghajanzadeh M, Hassanzadeh R, Alizadeh Sefat S, et al. Granulomatous mastitis: Presentations, diagnosis, treatment and outcome in 206 patients from the north of Iran. Breast 2015;24:456-60. [Crossref] [PubMed]
- Haddad M, Sheybani F, Arian M, Gharib M. Methotrexate-based regimen as initial treatment of patients with idiopathic granulomatous mastitis. Breast J 2020;26:325-7. [Crossref] [PubMed]
- Postolova A, Troxell ML, Wapnir IL, et al. Methotrexate in the Treatment of Idiopathic Granulomatous Mastitis. J Rheumatol 2020;47:924-7. [Crossref] [PubMed]
- Konan A, Kalyoncu U, Dogan I, et al. Combined long-term steroid and immunosuppressive treatment regimen in granulomatous mastitis. Breast Care (Basel) 2012;7:297-301. [Crossref] [PubMed]
- Raj N, Macmillan RD, Ellis IO, et al. Rheumatologists and breasts: immunosuppressive therapy for granulomatous mastitis. Rheumatology (Oxford) 2004;43:1055-6. [Crossref] [PubMed]
- Salesi M, Karimifar M, Salimi F, et al. A case of granulomatous mastitis with erythema nodosum and arthritis. Rheumatol Int 2011;31:1093-5. [Crossref] [PubMed]
- Vingerhoedt NM, Janssen S, Mravunac M, et al. Granulomatous lobular mastitis: a benign abnormality that mimics malignancy. Ned Tijdschr Geneeskd 2008;152:1052-6. [PubMed]
- Peña-Santos G, Ruiz-Moreno JL. Idiopathic granulomatous mastitis treated with steroids and methotrexate. Ginecol Obstet Mex 2011;79:373-6. [PubMed]
- National Institute for Health and Care Excellence. AZATHIOPRINE | Drug | BNF content published by NICE [Internet]. Bnf.nice.org.uk. 2020 [cited 18 March 2020]. Available online: https://bnf.nice.org.uk/drug/azathioprine.html#preTreatmentScreeningInformation
- Sparrow MP. Use of allopurinol to optimize thiopurine immunomodulator efficacy in inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2008;4:505-11. [PubMed]
- National Institute for Health and Care Excellence. ALLOPURINOL | Drug | BNF content published by NICE [Internet]. Bnf.nice.org.uk. 2022 [cited 20 April 2022]. Available online: https://bnf.nice.org.uk/interaction/allopurinol-2.html
- Adam L, Phulukdaree A, Soma P. Effective long-term solution to therapeutic remission in Inflammatory Bowel Disease: Role of Azathioprine. Biomed Pharmacother 2018;100:8-14. [Crossref] [PubMed]
Cite this article as: Hudson-Phillips SP, Beynon V, Houston A, Cosgrove C, Ho-Yen CM, Patel K, Tang S. Idiopathic granulomatous mastitis in pregnancy: a case report on a new management approach using azathioprine and allopurinol. AME Surg J 2023;3:30.



