
Liver function tests as predictors of choledocholithiasis: a scoping review
Highlight box
Key findings
• The included studies, examined parameters, and reported results have considerable heterogeneity and showed modest utility for LFTs in predicting choledocholithiasis with no consistent cut-off values or pattern identified.
What is known and what is new?
• What is known: LFTs are a commonly used predictor internationally for predicting choledocholithiasis.
• What is new: 19% and 14% of articles respectively reported bilirubin and other liver function tests to not be statistically significant predictors of choledocholithiasis.
What is the implication, and what should change now?
• Better defined cut-offs for LFTs as well as LFT trends for predicting choledocholithiasis need to be identified with further studies.
Introduction
Cholelithiasis is of increasing prevalence both in New Zealand and globally, with Middlemore Hospital facing over 750 acute admissions each year for cholelithiasis-related problems (1,2). A subset of patients with cholelithiasis will also have or develop choledocholithiasis, which impacts operative planning and contributes towards increased morbidity. Non-invasive techniques to diagnose choledocholithiasis include liver function tests (LFTs), abdominal ultrasound, magnetic resonance cholangiopancreatography (MRCP) and computed tomography (CT) cholangiography. Invasive techniques include endoscopic retrograde cholangiopancreatography (ERCP) and intraoperative cholangiogram (IOC) during cholecystectomy (3). Cost and resource constraints can limit access to advanced imaging such as MRCP, whilst invasive methods like the ERCP carry high risk of complications. As a result of said constraints, an emphasis has been placed on refining the diagnostic criteria and certainty of the more non-invasive, cost- and time-effective investigations like serum biochemistry. However, the accuracy and trend of LFTs to predict choledocholithiasis is not yet well defined with the American Society for Gastrointestinal Endoscopy (ASGE) guidelines, which categorizes any abnormality in LFTs as an intermediate risk of choledocholithiasis (at a rate of 10–50%) without defining these abnormalities any further (4). A scoping review looking at articles that report on a range of biochemical parameters (i.e., from a single liver enzyme, to up to six liver enzymes) was therefore conducted with the objective of investigating the utility of LFTs in predicting choledocholithiasis through a systematic review of current literature. This study is presented in accordance with the PRISMA-ScR reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-23-2/rc) (5).
Methods
Search strategy
A separate protocol was not drafted.
The following bibliographic databases were searched across the period of February to April 2022: PubMed, Medline and Scopus. The search strategy was collectively drafted by two independent reviewers, and further refined through wider team discussion and appraisal by the research supervisor.
The selected search strategy for PubMed identified retrospective and prospective cohort studies on adult patients in the English language, published from January 2011 to November 2021. The following search terms were used: liver function tests, liver test, liver enzymes, transaminases, serology, clinical liver enzymes, bilirubin, common bile duct (CBD) stones, bile duct obstruction, choledocholithiasis, calculous cholecystitis, ERCP, and MRCP. Initial search results were organised and screened via Zotero, a reference management software. Keyword screening, abstract review, and full-text review were conducted by two independent reviewers. Full-text reviews of articles were additionally completed by the supervisor if two independent reviewers were unable to reach consensus on article eligibility.
The following search strategy for the PubMed database was used:
(liver function tests[Title] OR liver test[Title] OR liver enzymes[Title] OR transaminases[Title] OR serology[Title] OR clinical liver enzymes[Title] OR bilirubin[Title] OR common bile duct stones[Title] OR bile duct obstruction[Title] OR choledocholithiasis[Title] OR calculous cholecystitis[Title] OR ERCP[Title] OR MRCP[Title])
Filters: Humans, Adult: 19+ years, from January 2011 to November 2021
Eligibility criteria
The inclusion criteria were: studies published between January 2011 and November 2021, studies that looked at patients age >19, and studies that reported at least one LFT as a predictor of choledocholithiasis.
The exclusion criteria were: full text not written in English, full text not locatable, studies lacking statistical data or had incomplete data tables, and studies focusing on a demographic and/or pathology outside the scope of this study (e.g., pregnancy, gallbladder malignancy).
Data extraction
The collection of data items from the included articles was performed using the following five themes: bilirubin, other LFTs outside of bilirubin, application of the ASGE Guidelines, repeat LFTs, and inflammatory markers (6). Statistical parameters of any biochemical predictors including sensitivity, specificity, positive predictive value, negative predictive value, and P values were extracted and reported as per the included studies but a quantitative meta-analysis was not performed. Data charting was done in duplicate, and was completed by three independent reviewers. The data charting was then reviewed by a head reviewer to assess for inconsistencies. On completion of data charting, the three independent reviewers collectively discussed results and resolved inconsistencies noted by the head reviewer.
Quality assessment (QA)
All studies were assessed using the National Institution of Health Quality Assessment (NIH QA) Tool for observational cohort and cross-sectional studies (7). Two assessors applied the same QA tool on all included articles, and a third assessor was involved when a given article produced QA scores that differed by more than two points.
Synthesis of results
The synthesis of results was completed according to the five data categories as listed in the “data extraction” section. This included identifying how many articles contained the data points for each respective data category, as part of our assessment of identifying the current available evidence in this field.
Results
Study characteristics
The selection process for this scoping review is demonstrated in Figure 1. A total of 2,786 articles were identified using the above search strategy, with 25 studies included after applying the eligibility criteria. The included studies gave a total sample size of 19,919 patients. Sixteen and eight studies were classified as retrospective and prospective respectively, with one study involving participants that were recruited both prospectively and retrospectively. Characteristics of the 25 eligible studies are summarised in Table 1.
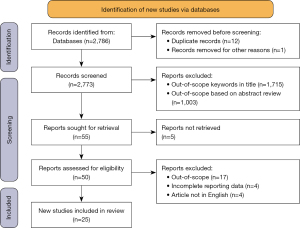
Table 1
| Name of first publishing author | Size of study population | Study location | Year of publication | Study type | Examined biochemical parameters | Quality assessment score† |
|---|---|---|---|---|---|---|
| Ahn (9) | 854 | Korea | 2016 | Retrospective | GGT, ALP, ALT, AST | 9 |
| Al-Jiffry (10) | 896 | Saudi Arabia | 2013 | Prospective | ALP | 6 |
| Bangaru (11) | 740 | USA | 2017 | Retrospective | ALP, ALT, AST | 9 |
| Björnsson (12) | 110 | Iceland | 2019 | Retro + prospective | GGT, ALP, ALT, AST | 9 |
| Chisholm (13) | 737 | USA | 2019 | Retrospective | ALP, ALT, AST, Lipase, WCC | 9 |
| He (14) | 2,724 | China | 2017 | Retrospective | ALT | 9 |
| Isherwood (15) | 195 | England | 2014 | Retrospective | ALP, AST | 6 |
| Jovanović (16) | 203 | Bosnia | 2011 | Prospective | GGT, ALP, ALT, AST, WCC | 7 |
| Kadah (17) | 344 | Israel | 2020 | Retrospective | GGT, ALP, ALT, AST, WCC | 7 |
| Kamath (18) | 275 | India | 2016 | Prospective | ALP, Amylase | 5 |
| Kang (19) | 196 | Korea | 2016 | Retrospective | ALP, ALT, AST | 8 |
| Lee (20) | 593 | Korea | 2019 | Retrospective | GGT, ALP, ALT, AST, WCC | 8 |
| Nárvaez Rivera (21) | 261 | Mexico | 2016 | Prospective | GGT, ALP, ALT, AST, WCC | 7 |
| Panda (22) | 152 | USA | 2018 | Retrospective | ALP, ALT, AST | 10 |
| Pejović (23) | 313 | Serbia | 2015 | Retrospective | GGT, ALP, ALT, AST, Amylase | 10 |
| Riggle (24) | 668 | USA | 2015 | Retrospective | ALT, AST, Lipase | 8 |
| Sethi (25) | 336 | USA | 2016 | Prospective | ALP, ALT, AST | 10 |
| Sherman (26) | 84 | USA | 2015 | Retrospective | GGT, ALP, ALT, AST, Amylase, Lipase | 10 |
| Song (27) | 424 | Korea | 2014 | Retrospective | GGT, ALP, ALT, AST, WCC | 8 |
| Stojadinov (28) | 154 | Serbia | 2015 | Prospective | ALP, ALT, AST, Amylase | 8 |
| Suarez (29) | 173 | USA | 2016 | Retrospective | ALT | 9 |
| Tozatti (30) | 254 | Brazil | 2015 | Retrospective | GGT, ALP, AST | 7 |
| Videhult (31) | 1,171 | Sweden | 2011 | Prospective | ALP | 7 |
| Yu (32) | 604 | USA | 2019 | Prospective | ALP, ALT, AST, Amylase, Lipase | 9 |
| Zgheib (33) | 7,458 | USA | 2021 | Retrospective | ALP, WCC | 8 |
†, NIH QA scores of 0–4 =poor; 5–10 =fair; 11–14 =good. GGT, gamma-glutamyl transferase; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; WCC, white cell count; NIH QA, National Institution of Health Quality Assessment.
QA
All of the included studies had a “fair” quality score (range, 5 to 10) as per the NIH QA Tool (Table 1) (7). Given that all articles underwent a full-text review as part of the selection process, articles that did not have a clearly defined research question and study population that were in line with the scope of this scoping review were excluded, thus eliminating articles of poorer quality ratings. None of the articles established a trend between time of presentation and time of biochemical analysis. The absence of follow-up as part of the study design for all the articles except one also limited most articles from obtaining higher quality scores.
Bilirubin
Twenty-one of the 25 articles commented on bilirubin as a predictor for choledocholithiasis. Three studies identified a bilirubin level of 1.8–4 mg/dL (31–68 µmol/L) to be a statistically significant predictor of choledocholithiasis (13,19,25). One article which specifically assessed bilirubin >4 mg/dL on initial serum biochemistry, reported this to be an independent predictor with an accuracy of 61.8% (29). Four additional articles identified bilirubin as a statistically significant predictor at the cut-offs of 1.2 mg/dL (21 µmol/L), 1.7 mg/dL (29 µmol/L), 2 mg/dL (34 µmol/L), and 2.1 mg/dL (36 µmol/L) respectively (9,18,23,33). A further five articles also reported statistically higher bilirubin levels in patients with choledocholithiasis, however specific cut-off levels were not identified (22,24,27,28,30). Seven studies found that bilirubin is a highly specific test to rule in choledocholithiasis (range, 0.73–0.97), but lacks sensitivity (range, 0.20–0.36) (14,17,19-21,29,30). Four studies found that bilirubin is not a statistically significant predictor (10,16,17,20). One study found that the association between increasing bilirubin and the risk of choledocholithiasis is not linear, i.e., a bilirubin of 1.8–4 mg/dL was found to be an independent predictor of choledocholithiasis, while a bilirubin of >4 mg/dL was not found to be an independent predictor (25).
Other LFTs outside of bilirubin
Twenty-two of the 25 articles commented on LFTs other than bilirubin. Three articles found that other LFTs were not statistically significant predictors for choledocholithiasis (19,21,25). Two of these articles looked at aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), with the third looking at all three in addition to gamma-glutamyl transferase (GGT).
The respective levels of LFTs that have been found to be statistically significant are summarised in Table 2.
Table 2
| Liver function test | Predictor cut-off level |
|---|---|
| GGT (IU/L) | >64, >224, >350 (11,15,19,26) |
| ALP (IU/L) | >100, >103, >108, >116, >120, >138, >190, >250, >400 (10,11,15,16,19,24,28,30,32,33) |
| AST (IU/L) | >40, >90, >106, >160 (10,15,16,24) |
| ALT (IU/L) | >102, >105, >320, ≥700, >750 (10,15,24,29,32) |
GGT, gamma-glutamyl transferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
GGT
GGT was found to be a statistically significant predictor in five articles (11,15,19,26,30). Two articles reported GGT to be the most reliable LFT for predicting choledocholithiasis, with one article reporting on GGT to be most sensitive predictor (9,17,30).
ALP
ALP was found to be a statistically significant predictor in twelve articles (10,11,13,15,16,18,19,24,28,30,32,33). One article found ALP to be the most specific and reliable LFT for predicting choledocholithiasis (30). Videhult et al. looked at bilirubin in conjunction to ALP and found that an elevated bilirubin and ALP had a positive predictive value of 42% (31).
Transaminases
AST and ALT were found to be statistically significant predictors in eight and eight articles respectively (10,13,15,16,18,24,29,30,32). Two articles specifically looked only at transaminases in the context of choledocholithiasis (11,12). Björnsson et al. and Bangaru et al. reported 8% and 6% of patients respectively that had transaminases >1,000 IU/L. Both studies found that patients of a younger age as well as patients that have had a prior cholecystectomy were more likely to have significant elevations in transaminases; patients of younger age tended to have smaller bile duct diameters, and the removal of the gall bladder resulted in a loss of reservoir for cholestatic pressure. Of the patients that had transaminases >1,000 IU/L in the Björnsson paper, 33% had undergone a prior cholecystectomy and the average CBD size was 7 mm. In comparison, Bangaru et al. reported 40% with prior cholecystectomies and an average CBD size of 8.5 mm. Bangaru et al. also reported that transaminase elevation in the context of choledocholithiasis was associated with a rapid decline within 24 to 72 hours of alleviating the obstruction.
Application of the ASGE guidelines
Ten of the 25 articles commented on the utility of the ASGE guidelines in predicting choledocholithiasis. The diagnostic accuracy of the application of the ASGE criteria for choledocholithiasis in high-risk patients ranged from 54.9–69.1%. This is concurrent with the guidelines, which state that patients that are stratified into the high-risk group have a greater than 50% incidence of choledocholithiasis. The diagnostic accuracy of the application of the ASGE criteria in intermediate-risk patients ranged from 28.8–40.0%, which is once again concurrent with the estimated 10–50% incidence of choledocholithiasis as stated in the guidelines. With regards to the low-risk criteria, specific figures surrounding its overall accuracy were not cited, however two studies concluded that the low-risk criteria and the subsequent recommendations regarding management are adequate, with He et al. quoting a negative predictive value of 90% (14). Additionally, there were four articles that proposed alternative scoring systems that had better diagnostic accuracies than the ASGE guidelines (13,16,17,26).
Repeat LFTs
Six of the 25 articles commented on the role of repeat LFTs in predicting choledocholithiasis. Two articles suggested that ongoing elevation of LFTs display significant correlation with choledocholithiasis, with one article concluding that a drop in liver function parameters on repeat tests is reflective of spontaneous passing of a CBD stone (9,15,22). Another article that attempted to quantify the change in LFTs in predicting spontaneous stone passage found that a drop in total bilirubin and ALT by at least 30% provided an overall accuracy of 48.5% (sensitivity 0.17, specificity 0.90) (29). Two articles concluded that serial LFTs would only identify a small proportion of new patients that were not identified with biochemical testing at initial presentation (14,29). One article concluded that increasing liver enzymes did not correlate with an increased risk of choledocholithiasis, and that patients with choledocholithiasis did not demonstrate any consistent patterns with regards to laboratory trends (32).
Inflammatory markers
Seven of the 25 articles looked at white cell count as a predictor for choledocholithiasis. Five of the articles found that white cell counts were not a statistically significant predictor (13,16,17,20,27). Two articles reported lower white cell counts in patients with choledocholithiasis (21,33).
Discussion
This scoping review aimed to identify which, if any, LFTs are able to reliably predict the presence of choledocholithiasis. The included studies, examined parameters, and reported results, had considerable heterogeneity and showed modest utility for LFTs in predicting choledocholithiasis with no consistent cut-off values or pattern identified.
There was much variation in the reported significance of bilirubin as a predictor of choledocholithiasis, and those that found significance gave no consistent cut-off value. Furthermore, the location of the respective study populations also determined how bilirubin was reported; American studies tended to study bilirubin using the parameters of 1.8–4 and >4 mg/dL as these are the cut-off values that are cited in the ASGE guidelines. On examining the three articles that reported bilirubin to not be a significant predictor, Al-Jiffry et al. involved a study population that is known to have high incidences of sickle cell anaemia and secondary polycythaemia due to high altitude (10). The pre-existing levels of high bilirubin at baseline within this population may have therefore created a skew in data when calculating bilirubin rise in the context of biliary disease. Lee et al. noted that a considerable proportion of its patients (39.8%) with elevated bilirubin up to >4 mg/dL had acute cholecystitis without choledocholithiasis (20). Further studies are thus warranted to identify the subset of patients with acute biliary disease and elevated bilirubin in the absence of choledocholithiasis. Jovanovic et al., who reported GGT to be the sole statistically significant biochemical parameter, did not include any further details surrounding population demographics or any additional data to help further assess the lack of statistical significance in bilirubin levels (16).
Similar to that of bilirubin, the results surrounding other LFTs as independent predictors also demonstrated a high degree of heterogeneity. Additionally, there are a number of studies that report on “other abnormal LFTs” without specifying which LFTs or their cut-off values, further contributing to the ambiguity of the use of other LFTs in diagnosing choledocholithiasis (20,21,25). There were three articles that commented on either ALP or GGT being the most reliable LFT in predicting choledocholithiasis, which supports the current understanding that disproportionate rises in GGT and ALP, relative to transaminases, can be interpretated as a cholestatic pattern (9,10,17). However, there were still a considerable proportion of articles that reported AST and ALT to be statistically significant predictors. Therefore, choledocholithiasis remains an important differential in patients with an elevated ALT and AST, particularly those of a younger age and having already undergone a cholecystectomy. A considerable number of studies excluded patients that had undergone prior cholecystectomy, which may explain why the finding of significant transaminase elevation secondary to CBD obstruction was not reported in the majority of studies.
On stratifying patients according to the ASGE guidelines, the reported rates of choledocholithiasis within each risk group were in-keeping with the stated probabilities from the guidelines. However, given that the application of the high-risk criteria will result in the performance of unnecessary ERCP in up to >40% of the patient population, some articles considered this to be suboptimal. Chisholm et al. stated that the acceptable negative ERCP rate according to a survey completed by gastroenterologists is approximately 25%, suggesting that there is still considerable room for improvement in the diagnostic accuracy of the ASGE guidelines before it can be more definitively used to guide clinical decision making (13).
A number of articles have proposed scoring systems which offer higher sensitivity, specificity and positive predictive values when compared to the ASGE guidelines. Sherman et al. demonstrated an overall accuracy of 88%, as well as a 100% positive predictive value in patients that have all five of the listed quantitative variables (CBD ≥9 mm, GGT ≥350 U/L, ALP ≥250 U/L, total bilirubin ≥3 mg/dL, direct (conjugated) bilirubin ≥2 mg/dL) (26). Kadah et al. proposed a predictive model based on three variables (age, CBD diameter, and GGT level) with an area under the receiver operating characteristic (ROC) curve of 0.73 (P<0.001) (17). Jovanović et al. proposed a similar predictive model based on three variables (CBD diameter, presence or absence of hyperechoic structure in the CBD as seen on abdominal ultrasound, and the presence of absence of elevated GGT) and an area under the ROC curve of 0.81 (P<0.001) (16). Lastly, Chisholm et al. proposed a predictive model comprising five variables (ALP >116 IU/L, ALT >105 IU/L, AST >90 IU/L, CBD diameter >6 mm, and total bilirubin >1.8 mg/dL), with an area under the ROC of 0.92 (13). All of these predictive models have higher accuracy rates than the ASGE guidelines, with all models either approaching or exceeding the acceptable threshold as reported in Chisholm et al. These models illustrate that a scoring tool to predict choledocholithiasis needs to incorporate radiological factors in addition to LFTs in order to improve predictive value.
There is some evidence to suggest that the additive value of repeat serum biochemistry towards improving diagnostic rates is minimal. However, biochemistry trends can help predict the spontaneous passage of a stone, suggesting that repeat LFTs may have a role in reducing rates of additional imaging.
There were a small number of articles that commented on the role of inflammatory markers in diagnosing choledocholithiasis. Nárvaez Rivera et al. reported lower white cell counts in patients with choledocholithiasis (10.4 vs. 11.8 K/µL), with an associated P value of 0.04 (21). Given the small difference in values, and the remaining studies showing no significance, there is little utility for white cell count in diagnosing choledocholithiasis.
This scoping review has several limitations which principally arise from heterogeneity of the included studies. This precluded a quantitative synthesis of the predictive data, therefore, a qualitative analysis was performed but this is similarly limited. All included studies were of moderate quality and varied in how choledocholithiasis was defined with some using the identification or presence of a definite stone as their definition, while others included the presence of sludge. Nine out of 25 studies were American and reported bilirubin cut-offs based on ASGE guidelines whereas other predictive models have used different cut-offs. Furthermore, this study specifically focuses on the utility of biochemical predictors in the diagnosis of choledocholithiasis whereas in clinical practice, the suspicion of choledocholithiasis relies on a combination of clinical, biochemical and radiological factors.
The overall findings of this scoping review are in-keeping with existing articles on this subject matter. Mongelli et al. concluded that whilst deranged LFTs are useful in assessing for the risk of choledocholithiasis, quantitative thresholds have yet to be established (34). Wang et al. similarly concluded that cut-offs for abnormal LFTs are not well defined and that the diagnostic performance of current scoring systems are inconsistent (4).
Conclusions
In conclusion, a clear and definitive pattern or trend of LFTs for predicting choledocholithiasis was not identified. Given the large amount of heterogeneity within the studied articles, further studies are warranted to better define the utility of individual LFTs, as well as repeat LFTs in diagnosing choledocholithiasis. The overall diagnostic performance of the ASGE guidelines is also suboptimal and suggests that existing guidelines on the risk stratification of patients with suspected choledocholithiasis needs to be reviewed. These could be further refined with cut-off values or changes in individual LFTs and combining with features on abdominal ultrasound such as CBD diameter in order to improve diagnostic accuracy, help omit costs and risks associated with more advanced imaging and ERCP, and ultimately reduce time to definitive operative management.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA-ScR reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-23-2/rc
Peer Review File: Available at https://asj.amegroups.com/article/view/10.21037/asj-23-2/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-23-2/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Acalovschi M, Lammert F. The Growing Global Burden of Gallstone Disease. World Gastroenterology Organisation. 2012;17(4). Available online: https://www.worldgastroenterology.org/publications/e-wgn/e-wgn-expert-point-of-view-articles-collection/the-growing-global-burden-of-gallstone-disease
- Poole GH, Jacobson AB, Hill AG. Index acute cholecystectomy: what's the problem?. ANZ J Surg 2018;88:1226-7. [Crossref] [PubMed]
- McNicoll C, Pastorino A, Farooq U, et al. Choledocholithiasis. StatPearls [Internet]. Florida: StatPearls Publishing; [updated 9 May 2022; cited 2 July 2022]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441961/
- Wang L, Mirzaie S, Dunnsiri T, et al. Systematic review and meta-analysis of the 2010 ASGE non-invasive predictors of choledocholithiasis and comparison to the 2019 ASGE predictors. Clin J Gastroenterol 2022;15:286-300. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [Crossref] [PubMed]
- ASGE Standards of Practice Committee. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc 2010;71:1-9. [Crossref] [PubMed]
- National Heart, Lung, and Blood Institute. Study Quality Assessment Tools. National Institutes of Health [Internet]. Maryland: National Heart, Lung, and Blood Institute; [updated July 2021; cited 2 July 2022]. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Haddaway N, Page M, Pritchard C, et al. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis Campbell Systematic Reviews. [updated June 2022; cited 3 May 2023]. Available online: https://doi.org/
10.1002/cl2.1230 - Ahn KS, Yoon YS, Han HS, et al. Use of Liver Function Tests as First-line Diagnostic Tools for Predicting Common Bile Duct Stones in Acute Cholecystitis Patients. World J Surg 2016;40:1925-31. [Crossref] [PubMed]
- Al-Jiffry BO, Elfateh A, Chundrigar T, et al. Non-invasive assessment of choledocholithiasis in patients with gallstones and abnormal liver function. World J Gastroenterol 2013;19:5877-82. [Crossref] [PubMed]
- Bangaru S, Thiele D, Sreenarasimhaiah J, et al. Severe Elevation of Liver Tests in Choledocholithiasis: An Uncommon Occurrence With Important Clinical Implications. J Clin Gastroenterol 2017;51:728-33. [Crossref] [PubMed]
- Björnsson HK, Björnsson ES. A significant proportion of patients with choledocholithiasis have markedly elevated alanine aminotransferase. Scand J Gastroenterol 2019;54:1155-9. [Crossref] [PubMed]
- Chisholm PR, Patel AH, Law RJ, et al. Preoperative predictors of choledocholithiasis in patients presenting with acute calculous cholecystitis. Gastrointest Endosc 2019;89:977-983.e2. [Crossref] [PubMed]
- He H, Tan C, Wu J, et al. Accuracy of ASGE high-risk criteria in evaluation of patients with suspected common bile duct stones. Gastrointest Endosc 2017;86:525-32. [Crossref] [PubMed]
- Isherwood J, Garcea G, Williams R, et al. Serology and ultrasound for diagnosis of choledocholithiasis. Ann R Coll Surg Engl 2014;96:224-8. [Crossref] [PubMed]
- Jovanović P, Salkić NN, Zerem E, et al. Biochemical and ultrasound parameters may help predict the need for therapeutic endoscopic retrograde cholangiopancreatography (ERCP) in patients with a firm clinical and biochemical suspicion for choledocholithiasis. Eur J Intern Med 2011;22:e110-4. [Crossref] [PubMed]
- Kadah A, Khoury T, Mahamid M, et al. Predicting common bile duct stones by non-invasive parameters. Hepatobiliary Pancreat Dis Int 2020;19:266-70. [Crossref] [PubMed]
- Kamath SU, Dharap SB, Kumar V. Scoring system to preoperatively predict choledocholithiasis. Indian J Gastroenterol 2016;35:173-8. [Crossref] [PubMed]
- Kang J, Paik KH, Lee JC, et al. The Efficacy of Clinical Predictors for Patients with Intermediate Risk of Choledocholithiasis. Digestion 2016;94:100-5. [Crossref] [PubMed]
- Lee HW, Song TJ, Park DH, et al. Diagnostic performance of the current risk-stratified approach with computed tomography for suspected choledocholithiasis and its options when negative finding. Hepatobiliary Pancreat Dis Int 2019;18:366-72. [Crossref] [PubMed]
- Nárvaez Rivera RM, González González JA, Monreal Robles R, et al. Accuracy of ASGE criteria for the prediction of choledocholithiasis. Rev Esp Enferm Dig 2016;108:309-14. [Crossref] [PubMed]
- Panda N, Chang Y, Chokengarmwong N, et al. Gallstone Pancreatitis and Choledocholithiasis: Using Imaging and Laboratory Trends to Predict the Likelihood of Persistent Stones at Cholangiography. World J Surg 2018;42:3143-9. [Crossref] [PubMed]
- Pejović T, Stojadinović MM. Scoring System Development and Validation for Prediction Choledocholithiasis before Open Cholecystectomy. Srp Arh Celok Lek 2015;143:681-7. [Crossref] [PubMed]
- Riggle AJ, Cripps MW, Liu L, et al. An analysis of omitting biliary tract imaging in 668 subjects admitted to an acute care surgery service with biochemical evidence of choledocholithiasis. Am J Surg 2015;210:1140-4; discussion 1144-6. [Crossref] [PubMed]
- Sethi S, Wang F, Korson AS, et al. Prospective assessment of consensus criteria for evaluation of patients with suspected choledocholithiasis. Dig Endosc 2016;28:75-82. [Crossref] [PubMed]
- Sherman JL, Shi EW, Ranasinghe NE, et al. Validation and improvement of a proposed scoring system to detect retained common bile duct stones in gallstone pancreatitis. Surgery 2015;157:1073-9. [Crossref] [PubMed]
- Song SH, Kwon CI, Jin SM, et al. Clinical characteristics of acute cholecystitis with elevated liver enzymes not associated with choledocholithiasis. Eur J Gastroenterol Hepatol 2014;26:452-7. [Crossref] [PubMed]
- Stojadinovic MM, Pejovic T. Regression tree for choledocholithiasis prediction. Eur J Gastroenterol Hepatol 2015;27:607-13. [Crossref] [PubMed]
- Suarez AL, LaBarre NT, Cotton PB, et al. An assessment of existing risk stratification guidelines for the evaluation of patients with suspected choledocholithiasis. Surg Endosc 2016;30:4613-8. [Crossref] [PubMed]
- Tozatti J, Mello AL, Frazon O. Predictor factors for choledocholithiasis. Arq Bras Cir Dig 2015;28:109-12. [Crossref] [PubMed]
- Videhult P, Sandblom G, Rudberg C, et al. Are liver function tests, pancreatitis and cholecystitis predictors of common bile duct stones? Results of a prospective, population-based, cohort study of 1171 patients undergoing cholecystectomy. HPB (Oxford) 2011;13:519-27. [Crossref] [PubMed]
- Yu CY, Roth N, Jani N, et al. Dynamic liver test patterns do not predict bile duct stones. Surg Endosc 2019;33:3300-13. [Crossref] [PubMed]
- Zgheib H, Wakil C, Al Souky N, et al. Liver function tests as predictors of common bile duct stones in acute cholecystitis patients with a chronic history: A retrospective cohort study on the ACS-NSQIP database. Medicine (Baltimore) 2021;100:e26885. [Crossref] [PubMed]
- Mongelli F, Di Giuseppe M, Porcellini I, et al. Liver Blood Tests in the Management of Suspected Choledocholithiasis. Lab Med 2021;52:597-602. [Crossref] [PubMed]
Cite this article as: Yuen WYR, Piteša R, McHugh T, Poole G, Singh PP. Liver function tests as predictors of choledocholithiasis: a scoping review. AME Surg J 2023;3:35.


