Use of homograft for recurrent pulmonary embolism due to popliteal venous aneurysm: a case report
Highlight box
Key findings
• Homograft interposition represents a valid treatment option for popliteal venous aneurysm when venorrhaphy or saphenous vein reconstruction is not feasible.
What is known and what is new?
• The surgical treatment ranges from aneurysmorrhaphy, to bypass and to interposition: for the latter two choices, the saphenous vein represents the graft most frequently used.
• When the saphenous vein is not available for any reasons, homograft represents a valid alternative.
What is the implication, and what should change now?
• We should consider the use of homograft also in venous patterns.
Introduction
Background
Venous aneurysm (VA) can be defined as an area of dilation 2 to 3 times the surrounding normal vein (1). They can be classified into two main groups: primary (congenital) or secondary (acquired) aneurysms, which arise from etiologies such as trauma, inflammation, degenerative processes, mechanical stress, and venous hypertension. They are rare and most are asymptomatic (2): an exception is represented by popliteal venous aneurysms (PVA) that could reveal themselves in the form of deep venous thrombosis (DVT), pulmonary embolism (PE) and death (3). Therefore, PVA are the most frequent and best-studied of all VAs. The treatment spectrum ranges from anticoagulation alone, to tangential aneurysmectomy with lateral venorrhaphy, aneurysm resection with primary anastomosis or interposition grafting (with saphenous vein or homograft) (4).
Rationale and knowledge gap
In scientific literature, we can’t find valid information regarding the use of homograft in venous pattern, so we used this “need situation” (lack of adequate saphenous vein) to observe the response of an interposition grafting with homograft.
Objective
With this case report, we want to demonstrate the safety and reliability of the homograft also in venous pattern. We present this case in accordance with the CARE reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-22-39/rc).
Case presentation
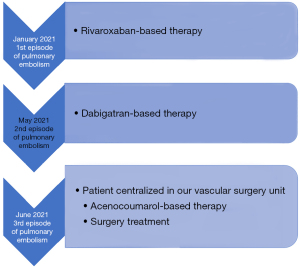
A 79-year-old female with a history of breast cancer under chemotherapy, epilepsy in medical therapy, previous saphenectomy of the right leg, recurrent urinary infections, arterial hypertension, and chronic anemia was admitted to the hospital, in January 2021, for worsening dyspnea. She underwent computed tomography (CT) angiography which confirmed the diagnosis of PE. She underwent negative echocardiography and duplex ultrasound (US) of the legs showing a saccular dilation of the left popliteal vein (reported 4.5 cm × 3 cm) with no evidence of superficial or DVT. A Rivaroxaban-based therapy (15 mg 1 tablet ×2) was then set up.
In May 2021, the patient was hospitalized again with the same symptoms: sudden dyspnea with a slight increase in cardiac enzymes. CT angiography once again confirmed the diagnosis of PE, echocardiography and US of the legs were negative for cardiac disease and DVT and a Dabigatran 150 mg twice daily-based therapy was set up.
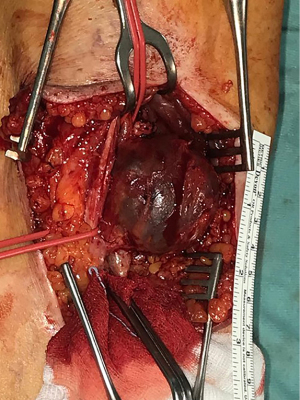
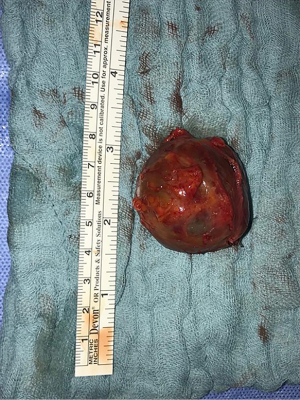
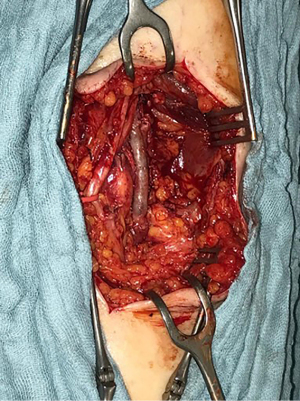
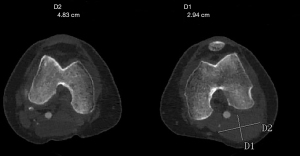
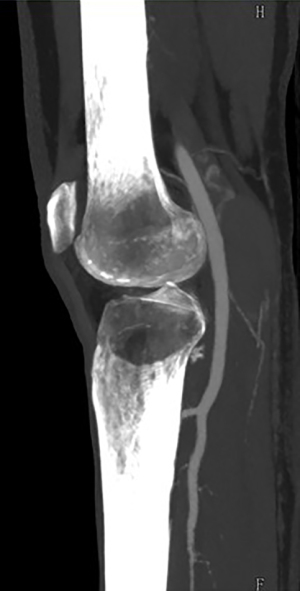
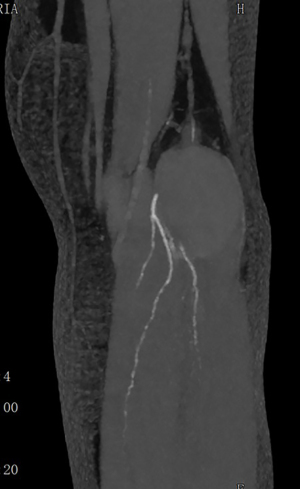
In June 2021, third episode of PE characterized by worsening dyspnea and left leg swelling. A multidisciplinary team was therefore created consisting of a radiologist, a hematologist, and a vascular surgeon. An Acenocoumarol 4 mg-based therapy was set up and the patient was then sent to our Vascular Surgery Unit (only after the third episode of PE). After a long discussion within the multidisciplinary team and with the patient, considering the lack of response to medical therapy, the decision was made to perform the surgery (Figure 1 summarizes the phases of the clinical case in chronological order). The patient underwent CT angiography of the legs for preoperative planning which confirmed the presence of a thrombosed saccular aneurysm of the left popliteal vein measuring 4.8 cm × 2.9 cm (Figures 2-4). As a first step we placed an inferior vena cava (IVC) filter (CelectTM Platinum Vena Cava Filter; COOK Medical LLC, Bloomington; Indiana, USA) to prevent further embolization during the surgical maneuvers, then we performed the aneurysm resection and the interposition with homograft, because we could not use an adequate great saphenous vein (previous saphenectomy of the right leg and small size of the left saphenous vein) (Figures 5-7). Due to the location and the anatomy of the aneurysm, we chose a posterior approach: the patient was placed prone on the operating table and we made an S-shaped incision (from the posterior medial aspect of the thigh and down the posterior lateral leg). At the end of the procedure, the IVC filter was removed. The postoperative course was regular. The patient was discharged on the fifth post-operative day, with Acenocoumarol 4 mg-based therapy [international normalized ratio (INR) range, 2.5–3] and compression stockings for a period of 6 months. Duplex US at day 15, day 30, day 90 and day 180 was performed with no evidence of deep vein thrombosis or graft stenosis.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Key findings
Homograft interposition represents a valid treatment option for PVA when venorrhaphy or saphenous vein reconstruction is not feasible.
Strengths and limitations
The patient was closely followed up and there were no evidence of graft thrombosis, stenosis or degeneration; obviously, it’s a single case and with an early follow-up.
Comparison with similar researches
There is not abundant literature on the management of VA, mainly due to its low incidence and to the lack of information available on the true prevalence which is estimated to be below 0.2% (5,6).
PVAs are more frequently associated with complications than aneurysms of the superficial venous system, such as thrombosis, PE, localized pain and swelling, more diffuse lower extremity swelling, and symptoms of chronic venous disease (7). As in this case, unfortunately, the first manifestation of VA is often represented by one of these complications.
In some cases, PVAs are misdiagnosed and misinterpreted as a Baker’s cyst (8). In a recently published case report, a 17-year-old female patient has developed painless swelling in the right popliteal fossa and was reported as a simple Baker’s cyst after US. After 4 years, she has developed a PE (successfully treated with medical therapy). This episode highlights the importance of using the Doppler Color flow whenever an anechoic mass is detected on US, in order to avoid misinterpreting the finding as a “cystic lesion”.
As well described in a recent retrospective multi-institutional review (5), the management of PVAs ranges from observation alone, to observation with anticoagulation or antiplatelet therapy, to surgical intervention. The surgical repair could be performed via aneurysmorrhaphy, bypass or interposition (Table 1): for the latter two choices, the saphenous vein represents the graft most frequently used. However, sometimes, the calibers of the native popliteal vein and autologous saphenous vein might not be compatible and for this reason, as described by Ito et al. it’s possible to use an autologous saphenous vein panel graft (ASVPG): this technique consists in harvesting the saphenous vein, dividing into segments, opening longitudinally and suturing side by side (10). It’s certainly a good option, especially to avoid synthetic grafts, because autologous veins are more resistant to infection and thrombosis.
Table 1
| Author | Year | Number of patients | Venorrhaphy | Aneurysmectomy | ||
|---|---|---|---|---|---|---|
| Primary anastomosis | Saphenous vein graft | Homograft | ||||
| Gillespie DL (1) | 1997 | 3 | 3 | – | – | – |
| Park JS (2) | 2011 | 1 | – | – | – | – |
| Johnstone JK (3) | 2015 | 8 | 5 | – | 3 | – |
| Sessa C (6) | 2000 | 25 | 19 | 2 | 4** | |
| Nasr W (7) | 2007 | 1 | 1 | – | – | – |
| Noppeney T (9) | 2019 | 39* | 27 | 2 | – | – |
| Gorgatti F | 2022 | 1 | – | – | – | 1 |
*, n=10 non-surgical treatment; **, resection with interposition of the greater saphenous vein (n=2), of the superficial femoral vein (n=1), resection with vein transposition (n=1).
Regarding patency, we don’t have valid information regarding the use of homograft in venous pattern. Instead, we have more information on the arterial district: Mezzetto et al. (11) analyzed 54 popliteal artery aneurysms treated with arterial cryopreserved homograft and reported a primary patency of 96.3%, 93.9%, and 88.3% at 12, 36, and 60 months, respectively; secondary patency of 98.1% at 12, 36, and 60 months.
In the present case, aneurysmorrhaphy was avoided due to the large diameter of the aneurysm and resection and interposition with homograft was preferred (adequate great saphenous vein not disponible). In particular, we used an arterial cryopreserved allograft: the strength of these allografts is their high resistance to infections and reduced intraoperative times. A possible and rare complication of arterial cryopreserved allografts after arterial reconstruction is late aneurysmal degeneration. The cause of this degeneration is to be found, both in the preparation and thawing of the grafts, and in the cell-mediated immune response towards the transplanted allograft (12). We currently have no literature after venous reconstruction.
We considered the possibility of popliteal vein thrombus shedding during the isolation maneuvers, therefore IVC filter implantation was performed before the open surgical approach.
According to the literature, most authors recommend anticoagulation after surgical correction of PVAs. Therefore, our patient received Acenocoumarol 4 mg for 6 months (INR range, 2.5–3). Follow-up after surgery showed excellent results and no anticoagulation therapy-related bleeding.
Explanations of findings
This experience allows us to say that homograft is a safe and valid alternative also in venous pattern but, before making a comparison with the saphenous vein, we have to collect more cases and do a longer follow-up.
Implications and actions needed
PVAs are very rare, and for this reason it could be very useful to increase the number of patients by involving other centers.
Conclusions
To our knowledge, this is the first case report in which a popliteal vein has been treated surgically using a homograft. This technique seems safe and reproducible. More patients and longer follow-up need to be analyzed to confirm the success of this case report.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-22-39/rc
Peer Review File: Available at https://asj.amegroups.com/article/view/10.21037/asj-22-39/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-22-39/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gillespie DL, Villavicencio JL, Gallagher C, et al. Presentation and management of venous aneurysms. J Vasc Surg 1997;26:845-52. [Crossref] [PubMed]
- Park JS, Kim SD, Park IY, et al. Popliteal vein aneurysm as a source of pulmonary embolism: report of a case and review of the world literature. Ann Vasc Surg 2011;25:1139.e9-12. [Crossref] [PubMed]
- Johnstone JK, Fleming MD, Gloviczki P, et al. Surgical treatment of popliteal venous aneurysms. Ann Vasc Surg 2015;29:1084-9. [Crossref] [PubMed]
- Patel R, Woo K, Wakefield TW, et al. Contemporary management and outcomes of peripheral venous aneurysms: A multi-institutional study. J Vasc Surg Venous Lymphat Disord 2022;10:1352-8. [Crossref] [PubMed]
- Bergqvist D, Björck M, Ljungman C. Popliteal venous aneurysm--a systematic review. World J Surg 2006;30:273-9. [Crossref] [PubMed]
- Sessa C, Nicolini P, Perrin M, et al. Management of symptomatic and asymptomatic popliteal venous aneurysms: a retrospective analysis of 25 patients and review of the literature. J Vasc Surg 2000;32:902-12. [Crossref] [PubMed]
- Nasr W, Babbitt R, Eslami MH. Popliteal vein aneurysm: a case report and review of literature. Vasc Endovascular Surg 2007;41:551-5. [Crossref] [PubMed]
- Krishan A, Droste JC, Molloy K, et al. Popliteal Vein Aneurysm Masquerading as a Baker's Cyst Leading to Pulmonary Embolism. Am J Med 2021;134:1495-8. [Crossref] [PubMed]
- Noppeney T, Kopp R, Pfister K, et al. Treatment of popliteal vein aneurysms. J Vasc Surg Venous Lymphat Disord 2019;7:535-42. [Crossref] [PubMed]
- Ito Y, Saito A, Shirai Y, et al. Surgical treatment of symptomatic popliteal vein aneurysm with autologous saphenous vein panel graft. J Vasc Surg Cases Innov Tech 2021;7:645-8. [Crossref] [PubMed]
- Mezzetto L, Scorsone L, Pacca R, et al. Treatment of popliteal artery aneurysms by means of cryopreserved homograft. Ann Vasc Surg 2015;29:1090-6. [Crossref] [PubMed]
- Touma J, Cochennec F, Parisot J, et al. In situ reconstruction in native and prosthetic aortic infections using cryopreserved arterial allografts. Eur J Vasc Endovasc Surg 2014;48:292-9. [Crossref] [PubMed]
Cite this article as: Gorgatti F, Pipitone MD, Zaraca F, Perkmann R, Coppi G. Use of homograft for recurrent pulmonary embolism due to popliteal venous aneurysm: a case report. AME Surg J 2023;3:40.