
Early experience with implementing enhanced recovery after surgery protocol during robot-assisted radical prostatectomy
Highlight box
Key findings
• Full enhanced recovery after surgery (ERAS) protocols have been safely implemented during robot-assisted radical prostatectomy (RARP).
What is known and what is new?
• The ERAS protocol is known to reduce operative complications and hospital stay, especially after gastrointestinal surgery. However, reports of urological surgeries are rare.
• There are few reports of RARP with ERAS in Japanese institutions. We implemented all but two ERAS items in the perioperative management of RARP. Among patients, 97% were successfully mobilized 3 h after surgery, and 100% started an early oral diet at 4 h after surgery.
What is the implication, and what should change now?
• Building a multidisciplinary ERAS team is mandatory for providing all ERAS items. Although the gastrointestinal organs are not involved in prostatectomy, the ERAS protocol can improve patient experience after RARP. Accumulation of experience with ERAS during RARP is required to validate our findings.
Introduction
Background
Enhanced recovery after surgery (ERAS) is an evidence-based perioperative care program aimed at reducing postoperative complications and recovery time after surgery (1). Initially implemented in colorectal surgery, the use of ERAS has expanded to almost all major surgeries in various organs (2-8). Shortened lengths of hospital stay, fewer perioperative complications, and financial benefits have been reported among various groups (9-11). In urological surgeries, however, the use of ERAS remains uncommon even in radical cystectomy with urinary diversion utilizing the small intestine. ERAS implementation during robot-assisted radical prostatectomy (RARP) is even rarer.
Typical ERAS protocols include >20 elements (12). The major elements include preoperative counseling, carbohydrate loading before surgery, early postoperative mobilization, minimally invasive surgery, and early resumption of drinks and foods after surgery.
Rationale and knowledge gap
We recently established a multidisciplinary perioperative ERAS program during RARP, at the NTT Medical Center Tokyo. Conventionally, patients undergoing RARP are instructed to fast from the night before surgery until postoperative day (POD) 1. In addition, the patients remained in their beds until noon on POD 1. In this study, we aimed to confirm the safety of early mobilization and food intake after RARP using the ERAS protocol.
Objective
Here, we describe our early experience in implementing a multidisciplinary ERAS program during the RARP. This study aimed to verify the safety and feasibility of the ERAS protocol in RARP management. This article is presented in accordance with the STROBE reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-23-4/rc).
Methods
Patients
Consecutive 105 patients with organ-confined prostate cancer who underwent RARP in the ERAS program between June 2021 and August 2022 at NTT Medical Center Tokyo were included in this observational study. The exclusion criteria were intestinal injury, high bleeding risk, and prolonged operating time (Figure 1). Written informed consent for publication was obtained from all the patients. The protocol for this research project was approved by the Ethics Committee of NTT Medical Center Tokyo (Approval No. 21-11) and conformed to the provisions of the Declaration of Helsinki (as revised in 2013).
ERAS program
Multidisciplinary ERAS team
Our ERAS team consisted of eight urologists, 10 anesthesiologists, more than 10 physical therapists, two registered dietitians, nurses, pharmacists, diabetologists, and clinical care pathway committee members. The roles of team members are presented in Figures 2,3 and Table 1. A diabetologist joined the ERAS team to assess the risk of hyperglycemia in patients with diabetes after carbohydrate administration. Although specific training for the ERAS protocol is not necessary, members share its concepts and goals before implementing it.
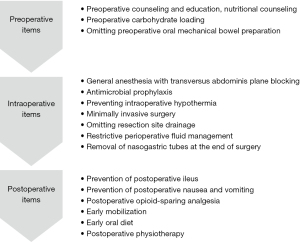
Table 1
| ERAS item | Summary |
|---|---|
| Preoperative | |
| 1 Preoperative counseling and education | Surgical details, hospital stay, and discharge criteria in oral and written form |
| 2 Preoperative medical optimization | Preoperative optimization of medical conditions and nutritional support |
| 3 Prehabilitation | Preoperative pelvic floor muscle training or exercise to improve general condition. Omitted due to lack of coverage by the Japanese medical treatment fee insurance |
| 4 Oral mechanical bowel preparation | Avoidance of laxatives |
| 5 Preoperative carbohydrate loading | 45 mg carbohydrate 2 h and 12 h before operation |
| 6 Preoperative fasting | Clear fluid intake until 2 h and solid foods until 6 h before general anesthesia induction |
| 7 Preanesthesia medication | Avoidance of long-acting sedatives |
| 8 Thrombosis prophylaxis | Pharmacological prophylaxis with LMWH. Not applied |
| Intraoperative | |
| 9 Epidural analgesia | Not applied. Transversus abdominis plane block was applied |
| 10 Minimally invasive approach | Robotic-assisted radical prostatectomy |
| 11 Resection site drainage | Perianastomotic and/or pelvic drain. Omitted |
| 12 Antimicrobial prophylaxis and skin preparation | Cefazolin sodium 1 g within 30 min before operation |
| 13 Standard anesthetic protocol | air-oxygen-desflurane, remifentanil, fentanyl, and rocuronium bromide |
| 14 Perioperative fluid management | Limited to less than 3 mL/kg/h |
| 15 Preventing intraoperative hypothermia | Bairhugger® |
| Postoperative | |
| 16 Nasogastric intubation | Removed at the end of the operation |
| 17 Urinary drainage | Placed for 6 days |
| 18 Prevention of postoperative ileus | Chewing gum every 3 h starting 3 h postoperatively |
| 19 Prevention of PONV | Administration of 6.6 mg dexamethasone intravenously |
| 20 Postoperative analgesia | Acetaminophen 1,000 mg every 6 h for 24 h |
| 21 Early mobilization | 3 h postoperatively |
| 22 Early oral diet | 4 h postoperatively |
| 23 Audit | Audit for protocol compliance and outcomes. Not applied |
ERAS, enhanced recovery after surgery; PONV, postoperative nausea and vomiting; LMWH, low molecular weight heparin.
ERAS items
ERAS items for the preoperative, intraoperative, and postoperative settings are shown in Figure 3 and consist of the following.
Preoperative items
(I) Preoperative counseling and education and nutritional counseling
Urologists, anesthesiologists, and nationally certified nutritionists provided preoperative counseling in outpatient settings. According to the European Society for Clinical Nutrition and Metabolism guidelines, the patients were advised to maintain a normal diet until the night before surgery (4). The patients were provided with a document regarding their expected recovery after surgery. Preoperative morbidities were optimized where possible.
(II) Prehabilitation physiotherapy
Prehabilitation, including the introduction of pelvic floor muscle training, was not performed because it was not covered by Japanese medical treatment fee insurance.
(III) Preoperative carbohydrate loading
Patients were administered 250 mL of carbohydrate fluid (Arginaid Water®, Nestle Health Science, Tokyo, Japan: 100 kcal, 22.5 g carbohydrate with 2.5 g arginine per 125 mL) at 2 and 12 hours, preoperative. Carbohydrate loading was administered to the patients diagnosed with diabetes mellitus under the supervision of a diabetologist.
(IV) Preoperative oral mechanical bowel preparation
No oral mechanical bowel preparation was performed. Twenty-four mg of sennoside was administered the night before the surgery.
Intraoperative items
(I) Anesthesia
General anesthesia was induced using propofol, fentanyl, remifentanil, and rocuronium and maintained using air-oxygen-desflurane, remifentanil, fentanyl, and rocuronium bromide. The total amount of fentanyl was restricted to 5 µg/kg. Intravenous acetaminophen was administered before the end of surgery. For postoperative analgesia, an ultrasound-guided transversus abdominis plane block or rectus sheath block with 0.25% ropivacaine was utilized to prevent areas of subcutaneous emphysema.
(II) Antimicrobial prophylaxis
One mg of cefazolin sodium was intravenously injected 30 min before the skin incision. Thereafter, the same amount of cefazolin sodium was injected every 12 h for two days.
(III) Preventing intraoperative hypothermia
Warming blankets (Full Body Bair Hugger™, 3M Japan, Tokyo, Japan) were used to prevent hypothermia during the operation.
(IV) Minimally invasive surgery
RARPs were performed by experienced urologists certified by Japanese Urological Association using the transperitoneal approach and Davinci Xi® (Intuitive Surgical Ltd., CA, USA). The lateral pedicles were cut using vessel-sealing systems (Ligasure™, Medtronic Japan, Tokyo, Japan). Unilateral nerve sparing was performed in selected patients, and Tacosil (CSL Behring, Tokyo, Japan) was used for hemostasis. We performed a Rocco stitch for posterior reconstruction. The bladder necks were preserved, and bidirectional running vesicourethral anastomosis was performed with double ended 3-0 MONOCRYL® (ETHICON, NJ, USA). Urethral catheters (16 Fr) were removed on POD 6.
(V) Resection site drainage
Resection site drainage was safely omitted.
(VI) Restrictive perioperative fluid management
Intraoperative IV fluid administration was limited to 3 mL/kg/h and included Ringer’s bicarbonate solution, antibiotics, and acetaminophen.
(VII) Nasogastric intubation
Nasogastric tubes were removed at the end of the surgery.
Postoperative items
(I) Urethral drainage
A urethral catheter (16 Fr) was placed in the bladder until removal on POD 6.
(II) Prevention of postoperative ileus
To prevent postoperative ileus, patients were encouraged to chew gum every 3 h, starting 3 h after surgery. Oral magnesium oxide (300 mg) was administered thrice daily until POD 7.
(III) Prevention of postoperative nausea and vomiting
To prevent postoperative nausea and vomiting, 6.6 mg dexamethasone was administered intravenously during anesthesia induction.
(IV) Postoperative opioid-sparing analgesia
For postoperative analgesia, patients were intravenously administered 1,000 mg of acetaminophen every 6 h for 24 h, followed by oral administration of 200 mg of celecoxib every 12 h.
(V) Early mobilization
The patients walked 20 m 3 h after surgery. The physical therapist assessed the patients’ conditions and canceled early mobilization if their vital signs deviated from the safety ranges. The safety range was set as follows: systolic blood pressure less than 180 mmHg; pulse rate of 50–120 beats/min; percutaneous oxygen saturation >90%. Early mobilization was also canceled when blood pressure dropped by more than 20% between the supine and sitting positions or between the sitting and standing positions. If the first mobilization trial failed, a second trial was performed 30 min later. Patients were encouraged to be out of bed for 2 h on the day of surgery. On POD 1, patients were encouraged to walk 100 m and attempt to be out of bed for 6 h. On POD 2, patients were encouraged to walk >300 m, assisted by physical therapists. On POD 3 and later, the patients were encouraged to exercise on a treadmill for 30 min/day.
(VI) Early oral diet
Patients were allowed to take clear fluid and CalorieMate Jelly® (Otsuka Pharmaceutical, Tokyo, Japan) 3 and 4 h after surgery, respectively. Normal meals were provided in the evening of the day of surgery.
Statistical analysis
Continuous variables were described as medians with interquartile ranges (IQR), and categorical variables were described as frequencies and percentages.
Results
Surgical results
Patient characteristics are described in Table 2. The median operative time, console time, and estimated intraoperative blood loss were 163 min, 121 min, and 40 mL, respectively (Table 3).
Table 2
| Variables | Cases (n=105) |
|---|---|
| Age, years, median [IQR] | 71 [63–75] |
| BMI, kg/m2, median [IQR] | 23 [20–25] |
| PSA, ng/mL, median [IQR] | 7 [5–11] |
| Gleason grade, n (%) | |
| 1 | 6 (5.7) |
| 2 | 29 (27.6) |
| 3 | 37 (35.2) |
| 4 | 14 (13.3) |
| 5 | 19 (18.1) |
| Clinical T stage, n (%) | |
| cT1 | 31 (29.5) |
| cT2 | 64 (61.0) |
| cT3 | 10 (9.5) |
| Charlson Comorbidity index, n (%) | |
| 0 | 79 (75.2) |
| 1 or more | 26 (24.8) |
IQR, interquartile range; BMI, body mass index; PSA, prostate specific antigen.
Table 3
| Variables | Cases (n=102) |
|---|---|
| Operation time, minutes, median [IQR] | 163 [148–186] |
| Console time, minutes, median [IQR] | 121 [108–150] |
| eBL, mL, median [IQR] | 40 [20–96] |
| Prostatic weight, g, median [IQR] | 40 [34.5–47.5] |
| Nerve sparing surgery, n (%) | |
| Yes | 32 (31.4) |
| No | 70 (68.6) |
| Intraoperative fluid infusion volume, mL, median [IQR] | 610 [510–750] |
| Length of postoperative hospital stay, n (%) | |
| 8 days | 85 (83.3) |
| 9 days and more | 17 (16.7) |
IQR, interquartile range; eBL, estimated blood loss.
Success rate of early mobilization after surgery
The ERAS protocol was successfully implemented in 102 (97.1%) patients after RARP. Three patients with intraoperative complications (prolonged operative time, small intestinal injury, or severe bleeding during nerve-sparing surgery) were excluded from the analysis. Among the 102 patients in whom the ERAS protocol was implemented, 99 (97%) were successfully mobilized from their beds and walked 20 m 3 h after surgery. Early oral intake was successful at 4 h after surgery in all patients. Two patients with orthostatic hypotension and nausea and one patient with tachycardia secondary to paroxysmal atrial fibrillation (heart rate: 150–170 beats per minute) failed to mobilize. Notably, none of the patients failed to mobilize due to postoperative pain.
Length of postoperative hospital stay
The postoperative hospitalization period was set to eight days in our clinical management plan. The median length of the postoperative hospital stay was 8 days. The hospital stay of 17 patients was extended because of postoperative complications such as urinary retention or leakage of urine from the vesicourethral anastomosis.
Discussion
Key findings
We developed a multidisciplinary team and implemented the ERAS protocol for 102 patients who underwent RARP between June 2021 and August 2022 at the NTT Medical Center Tokyo. In the current study, we adopted all ERAS items in the perioperative management of RARP except prehabilitation and patient audits. To adopt the above-mentioned ERAS items, the participation of urologists, anesthesiologists, physical therapists, nurses, and registered dietitians is essential. The ERAS protocol was safely implemented in the perioperative management of RARP, and 97% of patients were able to mobilize 3 h after surgery. The main reason for the failure to mobilize was orthostatic hypotension.
Strength and limitations
The strength of our study is that we implemented all but two ERAS items during RARP. Not all ERAS items have been adopted by institutions in the real world. For example, in a study reporting the favorable effect of ERAS on postoperative abdominal symptoms, preoperative carbohydrate loading and early resumption of an oral diet were not properly adopted (13). Among the ERAS items, the lowest adoption rates were reported for preoperative nutrition counseling, preoperative pelvic floor physiotherapy, early initiation of nutrition, and postoperative patient audits for further quality improvement (14). In our study, we did not introduce preoperative pelvic floor physiotherapy because of the lack of coverage by the Japanese medical treatment fees.
This study had some limitations. First, we enrolled a relatively small number of patients at a single institution. Second, this was not a comparative study. A comparative study with a larger number of patients is required to confirm the safety of ERAS during RARP in Japan.
Comparison with similar research
Same-day discharge RARP was recently reported in the United Kingdom and Australia (15,16). They used the ERAS protocol for the perioperative management of RARP, and the success rates for same-day discharge were 93.8% and 100% in the United Kingdom and Australia, respectively. The success rate of ERAS protocol was 100% in these studies (15,16). Another study assessed same-day discharge RARP with ERAS and prehabilitation pathway. They found that the implementation of same-day RARP in the context of ERAS and prehabilitation pathway is safe and reduces costs by 10.8% (17). To the best of our knowledge, there are two reports on RARP with ERAS from Japanese hospitals; however, only a few ERAS items were adopted in those studies (13,18).
Explanations of findings
In this cohort, 100% of patients succeeded in commencing an oral diet, and 97% were successfully mobilized 3 h after RARP. Conventionally, patients remained in bed until noon the following day. Meals were provided from lunch to POD 1. After the introduction of ERAS, patients could leave their beds and eat a normal diet on the day of surgery. In addition, a scheduled dose of acetaminophen effectively removed postoperative pain, resulting in successful early mobilization after surgery. Therefore, our patients’ experiences differed significantly from those of the patients with the conventional perioperative management method. Importantly, early mobilization and resumption of a regular diet are known to contribute to a shorter hospital stay (19).
Implications and actions needed
The use of the ERAS protocol remains uncommon in Japan. There are several reasons for the low utilization of ERAS in Japan. First, the hospital stay after surgery is determined according to the Diagnosis Procedure Combination/Per-Diem Payment System, which makes it difficult for hospitals to enjoy the financial benefits of shortening their postoperative hospital stay (1). Second, healthcare workers are still unfamiliar with multidisciplinary team care. The implementation of these new practices is difficult. The willingness to change to ERAS and the formation of multidisciplinary teams with good communication and collaboration are important for the successful implementation of ERAS (20,21). The standardization of order sets and care processes by establishing a clinical care pathway also supports the smooth implementation of the ERAS (21). Furthermore, attempts to conduct prospective clinical research through the introduction of a new perioperative management method helped to successfully implement ERAS for RARP in our case.
Conclusions
In conclusion, we showed that the ERAS protocol can be safely implemented for the perioperative management of RARP. Further prospective comparative studies are necessary to verify the safety and importance of the ERAS protocol for RARP.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-23-4/rc
Data Sharing Statement: Available at https://asj.amegroups.com/article/view/10.21037/asj-23-4/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-23-4/coif). MN serves as an unpaid editorial board member of AME Surgical Journal from January 2023 to December 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent for publication was obtained from all the patients. The protocol for this research project was approved by the Ethics Committee of NTT Medical Center Tokyo (Approval No. 21-11) and conformed to the provisions of the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Azhar RA, Bochner B, Catto J, et al. Enhanced Recovery after Urological Surgery: A Contemporary Systematic Review of Outcomes, Key Elements, and Research Needs. Eur Urol 2016;70:176-87. [Crossref] [PubMed]
- Cerantola Y, Valerio M, Persson B, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS(®)) society recommendations. Clin Nutr 2013;32:879-87. [Crossref] [PubMed]
- Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 2012;31:783-800. [Crossref] [PubMed]
- Lassen K, Coolsen MM, Slim K, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 2012;31:817-30. [Crossref] [PubMed]
- Melloul E, Hübner M, Scott M, et al. Guidelines for Perioperative Care for Liver Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J Surg 2016;40:2425-40. [Crossref] [PubMed]
- Nelson G, Bakkum-Gamez J, Kalogera E, et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations-2019 update. Int J Gynecol Cancer 2019;29:651-68. [Crossref] [PubMed]
- Nygren J, Thacker J, Carli F, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 2012;31:801-16. [Crossref] [PubMed]
- Thorell A, MacCormick AD, Awad S, et al. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J Surg 2016;40:2065-83. [Crossref] [PubMed]
- Porteous GH, Neal JM, Slee A, et al. A standardized anesthetic and surgical clinical pathway for esophageal resection: impact on length of stay and major outcomes. Reg Anesth Pain Med 2015;40:139-49. [Crossref] [PubMed]
- Madani A, Fiore JF Jr, Wang Y, et al. An enhanced recovery pathway reduces duration of stay and complications after open pulmonary lobectomy. Surgery 2015;158:899-908; discussion 908-10. [Crossref] [PubMed]
- Jeong O, Ryu SY, Park YK. Postoperative Functional Recovery After Gastrectomy in Patients Undergoing Enhanced Recovery After Surgery: A Prospective Assessment Using Standard Discharge Criteria. Medicine (Baltimore) 2016;95:e3140. [Crossref] [PubMed]
- Adamina M, Kehlet H, Tomlinson GA, et al. Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery 2011;149:830-40. [Crossref] [PubMed]
- Hori T, Makino T, Fujimura R, et al. Favorable Impact on Postoperative Abdominal Symptoms in Robot-assisted Radical Prostatectomy Using Enhanced Recovery After Surgery Protocol. Cancer Diagn Progn 2022;2:247-52. [Crossref] [PubMed]
- Liakos N, Beyer B, Ohlmann C, et al. Potential for optimizing the perioperative care in robotic prostatectomy patients by adoption of enhanced recovery after surgery principles. J Robot Surg 2022;16:415-9. [Crossref] [PubMed]
- Mulholland C, Soliman C, Furrer MA, et al. Same day discharge for robot-assisted radical prostatectomy: a prospective cohort study documenting an Australian approach. ANZ J Surg 2023;93:669-74. [Crossref] [PubMed]
- Hill GT, Jeyanthi M, Coomer W, et al. Same-day discharge robot-assisted laparoscopic prostatectomy: feasibility, safety and patient experience. BJU Int 2023;132:92-9. [Crossref] [PubMed]
- Ploussard G, Almeras C, Beauval JB, et al. Same-day discharge surgery for robot-assisted radical prostatectomy in the era of ERAS and prehabilitation pathways: a contemporary, comparative, feasibility study. World J Urol 2022;40:1359-65. [Crossref] [PubMed]
- Sugi M, Matsuda T, Yoshida T, et al. Introduction of an Enhanced Recovery after Surgery Protocol for Robot-Assisted Laparoscopic Radical Prostatectomy. Urol Int 2017;99:194-200. [Crossref] [PubMed]
- Maessen J, Dejong CH, Hausel J, et al. A protocol is not enough to implement an enhanced recovery programme for colorectal resection. Br J Surg 2007;94:224-31. [Crossref] [PubMed]
- Ament SM, Gillissen F, Moser A, et al. Identification of promising strategies to sustain improvements in hospital practice: a qualitative case study. BMC Health Serv Res 2014;14:641. [Crossref] [PubMed]
- Pearsall EA, Meghji Z, Pitzul KB, et al. A qualitative study to understand the barriers and enablers in implementing an enhanced recovery after surgery program. Ann Surg 2015;261:92-6. [Crossref] [PubMed]
Cite this article as: Nakamura M, Muraki Y, Tsuji Y, Watanabe J, Okabe K, Yamada Y, Kashiwagi M, Kiuchi A, Izumi T, Tsuru I, Ono A, Amakawa R, Inatsu H, Inoue Y, Yoshimatsu T, Fukuda A, Hayashi M, Matsumoto S, Komatsu T, Kameyama S, Kume H, Shiga Y. Early experience with implementing enhanced recovery after surgery protocol during robot-assisted radical prostatectomy. AME Surg J 2023;3:33.




