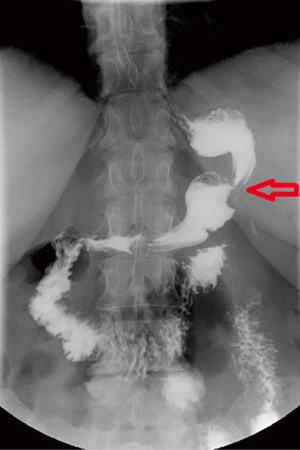
Sleeve gastrectomy technical parameters that may influence gastroesophageal reflux
Introduction
Obesity is a troublesome and worldwide growing pandemic disease (1). Bariatric surgery proved to be a very effective treatment regarding weight loss and obesity-related comorbidities control (2-4). Sleeve gastrectomy (SG) is one of the most performed procedures with a strong tendency of growth, perhaps because it is technically less challenging than the gold standard Roux-en-Y gastric bypass (RYGB) (5-7).
Comparing the procedures (SG vs. RYGB), good efficiency of both is observed in relation to the percentage and maintenance of long-term weight loss, as well as obesity-related comorbidities control (8-10). However, gastroesophageal reflux disease (GERD) after SG is the main drawback of this procedure. Anatomical and physiological changes as consequences of SG should justify the concern for “de novo” GERD or worsening of previous GERD symptoms.
The prevalence of GERD in the Western world is high and varies between 10–20% of adults (11), increasing significantly in obese patients (12,13), in whom it may reach 70% (14). The literature is controversial about the incidence of GERD after SG, and surprisingly it can vary from 9% to 60% (9-11).
This review aims to bring an overview of SG technical parameters that may influence GERD and the need for more studies in the field.
GERD pathophysiology
GERD occur due to a disbalance between natural anti-reflux mechanisms that need to be continent to a transdiaphragmatic pressure gradient that forces gastric contents upwards.
Valvular mechanism
- Lower esophageal sphincter (LES): muscular structure that allows the passage of food in a coordinated way from the esophagus towards the stomach, but also prevents food, acid, and bile reflux back to the esophagus. An inefficient LES is found in most GERD patients, although, it is not a “sine qua non” condition for the development of reflux since it depends on the other mechanisms involved in the anti-reflux barrier (15,16).
- Diaphragm and phrenoesophageal membrane: the phrenoesophageal membrane is the continuation of the transversalis fascia, consisting in an elastic fiber compassing the esophagus. It transmits positive pressure on the distal part of the esophagus through the esophageal hiatus, composing the LES mechanism as an extrinsic component (15,17).
- Angle of His and Gubaroff valve: the angulation of the stomach close to the gastroesophageal junction (GEJ) prevents the rise of gastric contents towards the esophagus, increasing the distance between the gastric fundus where the alimentary bolus is laid up, and projecting the fundus toward the esophagus during gastric distention. Gubaroff valves works like a cushion mechanism of the mucosa, in the distal esophagus, in its transition to the stomach at the level of GEJ (15,16).
- Esophageal peristalsis: through synchronized contractions, they propel the food bolus in one direction from the esophagus to the stomach, in addition to acting on the esophageal “clearance” when physiological reflux occurs (16).
Transdiaphragmatic pressure gradient
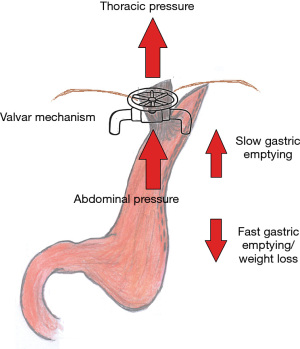
The thoracic pressure tends to be negative, promoting relative suction of the gastric contents, while the abdominal pressure tends to be positive, pushing the gastric contents towards the thorax. This gradient is normally balanced by natural valvular mechanisms. However, patients with obesity have greater abdominal pressure, in addition to an increase in the number of transient relaxations of the LES, which can generate an imbalance between these mechanisms, leading to GERD (13).
GERD may occur secondary to an inefficient valve at the GEJ and/or a high transdiaphragmatic pressure gradient surpassing the valve mechanism. Surgical procedures that change this balance should favor GERD.
GERD pathophysiology after SG
SG is a restrictive procedure based on the construction of a narrow gastric tube, providing changes in the angle of His that may favor the occurrence of GERD. Moreover, an increase in the intragastric pouch pressure, mostly attributed to different tube shapes such as excessive pouch narrowing or twists, that vary according to surgeon preferences and patients’ characteristics, may, theoretically, worse GERD symptoms. Finally, possible damage in the sling fibers of the LES during the stapling may favor a decrease in the basal pressure or transient relaxation of the LES. On the other hand, a decrease in the mass of parietal cells and a hasty gastric emptying in consequence to the gastrectomy, should be a defense against GERD (18) (Figure 1).
SG technical parameters to prevent GERD
- Saving sling fibers of the LES: some authors believe that stapling close to the GEJ should damage the muscular structure of LES, consequently increasing the number of transient LES relaxation, decreasing the basal pressure of the LES, and been responsible for the severity of GERD symptoms (19-22). Most of authors suggests that the stapling should be at least 1–2 cm far from the GEJ (19).
- Diaphragm and phrenoesophageal membrane dissection should favor the disruption of an important natural anti-reflux barrier, promoting the rising of hiatus hernia (HH) (19) that is commonly associated with the imbalance in the anti-reflux barrier. It is essential to carry out an accurate dissection to identify and close the esophageal hiatus when it is enlarged (19).
- Angle of His and Gubaroff valve: Petersen et al. demonstrated that the positioning of the stapler line close to the angle of His and without injuring the sling fibers or LES results in higher pressure at the His, consequently, higher LES pressure. On the other hand, a too-narrow stapling at the angle of His, can cause GERD symptoms (23). Moreover, the SG fundus resection causes the angle of His to become obtuse, which is associated with the pathophysiology of GERD (18).
- Gastric tube: the gastric pouch performed in SG adjusted by a tiny bougie, in addition to the fundus resection could promote a rising in the gastric tube pressure, beyond avoiding the pouch relaxation consequently to the abolishing of the post-feeding vasovagal reflex. Therefore, the decrease in gastric compliance and the rising in gastric pouch pressure should overcome the LES barrier favoring GERD (12,19,21). Moreover, a gastric stenosis or an exceedingly narrow SG could worsen postoperative GERD symptoms (Figure 2). Another concern is related to the tube twisting that can occur during the stapling, which will certainly rise the gastric tube pressure in addition to alimentary stasis (24). For that reason, the SG should be the widest at the antrum (5–6 cm from the pylorus) and the narrowest at the cardia. A retrospective study performed with 120 SG patients has demonstrated that using a 42-Fr bougie has better results on the incidence of GERD after the procedure when compared with a 32-Fr bougie. For the patients with a 42-Fr bougie, almost 80% reported postoperative improvement of GERD symptoms, compared with 60% of patients in the 32-Fr group. Further, GERD symptoms decreased postoperatively in 3% and 10% of the patients, respectively (25). Garay et al. had shown that the preservation of the antrum accelerates gastric emptying, reducing GERD by decreasing the intragastric pressure (26). Nevertheless, Hanssen et al. concluded that there is a relation between the gastric pouch volume and weight loss, seeming that SG tube ≥100 mL at 6 months is associated with poor weight loss (27). That makes controversial to perform the “perfect” SG.
- Weight loss and abdominal pressure: it is estimated that 50–60% of excessive weight loss is achieved after 1–2 years of SG (7,8). Weight loss is associated with decreasing in the abdominal pressure which could improve GERD symptoms thus to the reduction in the trans-diaphragmatic pressure (13). Thus, a narrow gastric tube, calibrated by a 32–36 Fr bougie, with a volume no more than 100 mL is essential to provide a satisfactory weight loss (27), and consequently a decrease in the abdominal pressure.
According to these assumptions some authors suggested key points to perform SG. Careful dissection of the angle of His, avoiding stapling too close to the GEJ, thus preserving the natural valve mechanism as much as possible. The tube must be wider in the antrum and more adjusted in the GEJ, to promote better transit of the food bolus and reduce gastric stasis, in addition to avoiding twisting of the tube during stapling that occurs with stenosis; calibrate the pouch by bougie 32–36 Fr and carry out the complete mobilization of the gastric fundus before the resection (28,29). It is important to evaluate the presence of a hiatal hernia and suture/close it when it is defective (6,8). Braghetto et al. could demonstrate a hiatal hernia incidence of 5% after SG in patients without HH previously (19). Although there is insufficient evidence in the literature, and weight loss should be a protect factor to hiatal hernia recurrence, a panel of specialists agreed that it is important to fix the HH when performing SG (28,30) (Figure 3).
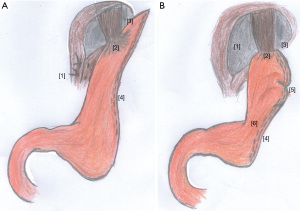
The literature large variation in the incidence of GERD after SG is intriguing. Chhabra et al. have demonstrated an important variation in SG key technical points when surgical SG videos were evaluated by peers, and could find a relation between the technique adopted and early complications (31). Probably the lack of SG standardization combined with technical difficulties inherent to patient’s characteristics may justify those findings.
Conclusions
SG can provide anatomical and physiological changes which should ultimately favor GERD. There are technical parameters when performing SG to avoid GERD. However, there is a lack of SG standardization in the literature which hinders the performance of the “perfect” SG tube. More studies are needed to put some light on the topic.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://asj.amegroups.com/article/view/10.21037/asj-23-11/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-23-11/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017;390:2627-42. [Crossref] [PubMed]
- Lo Menzo E, Szomstein S, Rosenthal RJ. Changing trends in bariatric surgery. Scand J Surg 2015;104:18-23. [Crossref] [PubMed]
- Melissas J, Braghetto I, Molina JC, et al. Gastroesophageal Reflux Disease and Sleeve Gastrectomy. Obes Surg 2015;25:2430-5. [Crossref] [PubMed]
- Kwok CS, Pradhan A, Khan MA, et al. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol 2014;173:20-8. [Crossref] [PubMed]
- Sharples AJ, Mahawar K. Systematic Review and Meta-Analysis of Randomised Controlled Trials Comparing Long-Term Outcomes of Roux-En-Y Gastric Bypass and Sleeve Gastrectomy. Obes Surg 2020;30:664-72. [Crossref] [PubMed]
- Gagner M. Is Sleeve Gastrectomy Always an Absolute Contraindication in Patients with Barrett's? Obes Surg 2016;26:715-7. [Crossref] [PubMed]
- Palermo M, Gagner M. Why We Think Laparoscopic Sleeve Gastrectomy Is a Good Operation: Step-by-Step Technique. J Laparoendosc Adv Surg Tech A 2020;30:615-8. [Crossref] [PubMed]
- Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ 2012;31:219-30. [Crossref] [PubMed]
- Peterli R, Wölnerhanssen BK, Vetter D, et al. Laparoscopic Sleeve Gastrectomy Versus Roux-Y-Gastric Bypass for Morbid Obesity-3-Year Outcomes of the Prospective Randomized Swiss Multicenter Bypass Or Sleeve Study (SM-BOSS). Ann Surg 2017;265:466-73. [Crossref] [PubMed]
- Mandeville Y, Van Looveren R, Vancoillie PJ, et al. Moderating the Enthusiasm of Sleeve Gastrectomy: Up to Fifty Percent of Reflux Symptoms After Ten Years in a Consecutive Series of One Hundred Laparoscopic Sleeve Gastrectomies. Obes Surg 2017;27:1797-803. [Crossref] [PubMed]
- Oor JE, Roks DJ, Ünlü Ç, et al. Laparoscopic sleeve gastrectomy and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Surg 2016;211:250-67. [Crossref] [PubMed]
- Patti MG, Schlottmann F. Gastroesophageal Reflux After Sleeve Gastrectomy. JAMA Surg 2018;153:1147-8. [Crossref] [PubMed]
- de Mello Del Grande L, Herbella FAM, Katayama RC, et al. Transdiaphragmatic Pressure Gradient (TPG) Has a Central Role in the Pathophysiology of Gastroesophageal Reflux Disease (GERD) in the Obese and it Correlates with Abdominal Circumference but Not with Body Mass Index (BMI). Obes Surg 2020;30:1424-8. [Crossref] [PubMed]
- Anand G, Katz PO. Gastroesophageal reflux disease and obesity. Rev Gastroenterol Disord 2008;8:233-9. [PubMed]
- Menezes MA, Herbella FAM. Pathophysiology of Gastroesophageal Reflux Disease. World J Surg 2017;41:1666-71. [Crossref] [PubMed]
- Herbella FA, Patti MG. Gastroesophageal reflux disease: From pathophysiology to treatment. World J Gastroenterol 2010;16:3745-9. [Crossref] [PubMed]
- Valezi AC, Herbella FA Jr, Mali J. Gastroesophageal reflux disease: pathophysiology. In: Fisichella PM, Allaix ME, Morino M, et al. editors. Esophageal diseases. Evaluation and treatment. Berlin: Springer; 2014:41-51.
- Nadaleto BF, Herbella FA, Patti MG. Gastroesophageal reflux disease in the obese: Pathophysiology and treatment. Surgery 2016;159:475-86. [Crossref] [PubMed]
- Braghetto I, Korn O. Late esophagogastric anatomic and functional changes after sleeve gastrectomy and its clinical consequences with regards to gastroesophageal reflux disease. Dis Esophagus 2019;32:doz020. [Crossref] [PubMed]
- Bou Daher H, Sharara AI. Gastroesophageal reflux disease, obesity and laparoscopic sleeve gastrectomy: The burning questions. World J Gastroenterol 2019;25:4805-13. [Crossref] [PubMed]
- Burgerhart JS, Schotborgh CA, Schoon EJ, et al. Effect of sleeve gastrectomy on gastroesophageal reflux. Obes Surg 2014;24:1436-41. [Crossref] [PubMed]
- Gorodner V, Buxhoeveden R, Clemente G, et al. Does laparoscopic sleeve gastrectomy have any influence on gastroesophageal reflux disease? Preliminary results. Surg Endosc 2015;29:1760-8. [Crossref] [PubMed]
- Petersen WV, Meile T, Küper MA, et al. Functional importance of laparoscopic sleeve gastrectomy for the lower esophageal sphincter in patients with morbid obesity. Obes Surg 2012;22:360-6. [Crossref] [PubMed]
- Dhorepatil AS, Cottam D, Surve A, et al. Is pneumatic balloon dilation safe and effective primary modality of treatment for post-sleeve gastrectomy strictures? A retrospective study. BMC Surg 2018;18:52. [Crossref] [PubMed]
- Spivak H. Laparoscopic sleeve gastrectomy using 42-French versus 32-French bougie. Obes Surg 2014;24:1095. [Crossref] [PubMed]
- Garay M, Balagué C, Rodríguez-Otero C, et al. Influence of antrum size on gastric emptying and weight-loss outcomes after laparoscopic sleeve gastrectomy (preliminary analysis of a randomized trial). Surg Endosc 2018;32:2739-45. [Crossref] [PubMed]
- Hanssen A, Plotnikov S, Acosta G, et al. 3D Volumetry and its Correlation Between Postoperative Gastric Volume and Excess Weight Loss After Sleeve Gastrectomy. Obes Surg 2018;28:775-80. [Crossref] [PubMed]
- Rosenthal RJ. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis 2012;8:8-19. [Crossref] [PubMed]
- Felinska E, Billeter A, Nickel F, et al. Do we understand the pathophysiology of GERD after sleeve gastrectomy? Ann N Y Acad Sci 2020;1482:26-35. [Crossref] [PubMed]
- Castagneto-Gissey L, Russo MF, D'Andrea V, et al. Efficacy of Sleeve Gastrectomy with Concomitant Hiatal Hernia Repair versus Sleeve-Fundoplication on Gastroesophageal Reflux Disease Resolution: Systematic Review and Meta-Analysis. J Clin Med 2023;12:3323. [Crossref] [PubMed]
- Chhabra KR, Thumma JR, Varban OA, et al. Associations Between Video Evaluations of Surgical Technique and Outcomes of Laparoscopic Sleeve Gastrectomy. JAMA Surg 2021;156:e205532. [Crossref] [PubMed]
Cite this article as: Katayama RC, Herbella FAM, Patti MG, Arasaki CH, Oliveira RO, de Grande AC. Sleeve gastrectomy technical parameters that may influence gastroesophageal reflux. AME Surg J 2023;3:46.