
A multi-disciplinary approach to gestational gigantomastia management: a case report
Highlight box
Key findings
• A 34-year-old woman G8P2 who was 18 weeks pregnant, presented to the emergency department with progressive gestational gigantomastia complicated by worsening bilateral breast skin necrosis.
What is known and what is new?
• The pathogenesis for gestational gigantomastia is poorly understood, but proposed risk factors are Caucasian race, multiparity, prior miscarriages, and autoimmune diseases.
• Definitive surgical intervention is a bilateral mastectomy. Bilateral breast reduction is not a durable treatment option as the breast tissue will continue to grow in the setting of pregnancy. Medical management with Bromocriptine while safe in pregnancy is generally unsuccessful. Gestational gigantomastia can occur after previous successful deliveries with no prior history.
What is the implication, and what should change now?
• Bilateral palliative mastectomy is safe for both the mother and fetus in the setting of gestational gigantomastia. Multidisciplinary management helps to optimize surgical timing to mitigate complications while optimizing both fetal and maternal outcomes.
Introduction
There has yet to be a current consensus definition for gestational gigantomastia as there are several definitions based on breast size. However, it occurs during pregnancy when the breasts expand quickly and disproportionately (1,2). A quantitative description is gross breast enlargement requiring the removal of more than 1,500 g of breast tissue (1). Although gestational gigantomastia has a reported frequency of 1:28,000–1:100,000 pregnancies, this may be underreported due to lack of training surrounding this rare condition (1).
Gestational gigantomastia occurs during the first or early in the second trimester and correlates closely to the period of high gonadotropin levels, which may suggest a potential hormonal etiology. The recommended management of gestational gigantomastia has evolved with advances in medicine, obstetrics, anesthesia, and surgery as better surgical techniques, investigative tools, and research work have allowed more understanding of the issue. Also advances in anesthesia and increased anesthesia provider comfort/skill in providing anesthesia during pregnancy. Previously, management involved the termination of pregnancy, however, surgery can be safely carried out during pregnancy with optimization of surgical timing to mitigate maternal and fetal harm (1). This report is a unique case of gestational gigantomastia in a 34-year-old Caucasian pregnant woman G8P2 with no previous history who presented after two successful previous deliveries and the impact of a multi-disciplinary approach to her and the baby’s chances of survival. We present this article in accordance with the CARE reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-23-13/rc).
Case presentation
A 34-year-old Caucasian woman G8P2 who was 18 weeks pregnant, presented to the emergency department in October 2022 with progressive gestational gigantomastia complicated by worsening bilateral breast skin necrosis (Figure 1). Her breast size had significantly increased from a C-cup to a P-cup, causing severe pain and an inability to ambulate. She was on progesterone for the first 12 weeks of her current pregnancy to prevent fetal loss. Past obstetric history was notable for multiple fetal losses from chromosomal abnormalities after two successful previous pregnancies with no prior episodes of gestational gigantomastia.
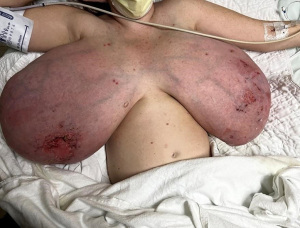
On examination, both breasts were grossly enlarged (left greater than right), with skin ulceration, erythema, skin thickening, and serous drainage bilaterally with no focal abscesses or collections (Figure 1). Bilateral breast ultrasound did not reveal any focal collections or masses. Given the patient’s open wounds and pain with breast manipulation, a diagnostic mammogram or biopsy was not possible. The patient’s incidental diagnostic mammogram finding 3 years prior was reported as Breast Imaging Reporting and Data System (BI-RADS) 3 with a likely benign finding, but the patient never presented for her interval follow-up imaging.
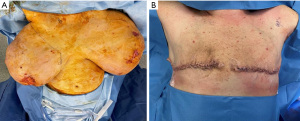
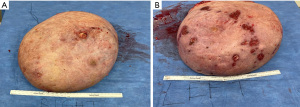
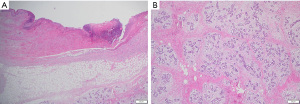
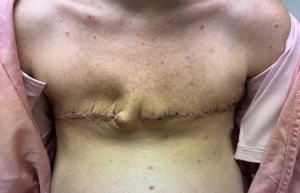
On presentation to the hospital, she was managed conservatively with intravenous antibiotics, bromocriptine, and local wound care. She failed to respond to nonsurgical therapies as anticipated with escalating opioid medication requirements, worsening skin necrosis, and progressive bilateral breast enlargement. A multidisciplinary team of medicine, obstetrics, breast surgery, and plastic surgery concluded that a simple bilateral mastectomy was the best option for the patient given severity of disease and lack of response to conservative therapy. Termination of pregnancy was not considered as the patient desired to keep the viable pregnancy and alternative treatment strategy of bilateral mastectomy was deemed feasible by the multidisciplinary team. Due to the high recurrence rates and her skin necrosis, the patient was not a good surgical candidate for reduction mammoplasty. Therefore, she underwent a bilateral palliative mastectomy on hospital day 7 (Figure 2) in December 2022. The patient tolerated the procedure well. Preoperative and postoperative fetal dopplers were confirmed as recommended based on gestational age of 19 weeks at surgical intervention. The patient was discharged on postoperative day 2 with standard post-mastectomy pain medication and bilateral breast drains. Breast pathology revealed benign tissue with stromal hyperplasia with the right breast weighing 9,150 grams and the left of 9,250 grams (Figure 3). The photomicrographs showed expansile lobular units with no histopathologic abnormality and associated ulcerated skin (Figure 4). She presented to her 1-week postoperative visit with a total resolution of her pain and mobility status (Figure 5). Her pregnancy progressed with a normal 20-week ultrasound, and she went on to have a successful delivery. Breast reduction was not offered due to likelihood of being unsuccessful with gestational gigantomastia as a result of its high recurrence rate with ongoing pregnancy. Breast reconstruction was not offered at the time of mastectomy given profound skin ulceration and concern for tissue expander or implant infection. The patient expressed a lack of interest in delayed breast reconstruction post-delivery but plastic surgery consultation was offered if desired.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Informed consent was obtained from the patient for the publication of this case report and accompanying images.
Discussion
Gestational gigantomastia commonly presents as a disproportionate increase of either one or, more commonly, both breasts with associated grossly enlarged areola and nipples, bulging superficial veins, and rarely infection, ulceration, and necrosis (1,3). Stasis in the lymphatic and venous systems can further increase the risk of developing ulcers, as exhibited in the index patient who presented with bilateral breast necrosis. The typical age of diagnosis is usually 16 to 35 years, with the majority being reported between 26 to 30 years (1,4). Apart from pregnancy, gigantomastia can be induced by medications and puberty. The pathogenesis and risk factors are not fully understood, but some patient factors have been identified. It occurs more commonly in Caucasians than Black patients (9:4). Prior gestational gigantomastia is the most likely predictor for gestational gigantomastia in a subsequent pregnancy; however, multiparity and history of autoimmune diseases have been suggested as proposed risk factors (1,5,6). Our patient shared certain personal and medical characteristics in keeping with previous studies. She was within the reported age group, female, Caucasian, multiparous, and experienced six previous miscarriages, which may have been due to potential autoimmune disease. The patient had a past medical history of primary biliary cholangitis cirrhosis of presumed autoimmune etiology, a proposed risk factor for gestational gigantomastia. The patient was currently at 18 weeks gestation of her 3rd viable pregnancy but experiencing her first episode of gestational gigantomastia. Her gestational gigantomastia was noted in the first half of her second trimester, which is consistent with the typical timing of presentation from previous case reports (1,3). Other studies have theorized that elevated hormone levels (especially prolactin) and/or increased hormone receptor levels have been postulated to be possible etiologies for gestational gigantomastia. However, some reported cases have a normal hormone profile (1). The index patient had no abnormal hormone levels documented consistent with some reported cases. However, she did use progesterone for the first 12 weeks of this pregnancy (1).
Both the mother and the fetus may experience a range of complications due to gestational gigantomastia, including skin ulceration, infection, necrosis, shoulder pain, back pain, and postural instability (1,7), all of which were present in this patient. Rarely gestational gigantomastia cases experience severe sepsis, renal impairment, multiorgan dysfunction syndrome, and even death (1,7). Palliative mastectomy was pursued in this patient to avoid these more serious secondary complications. Gestational gigantomastia is associated with severe societal, emotional, and social debility (3). Due to the complexity of gestational gigantomastia, a multidisciplinary team is often essential to ensure all aspects of patient and fetal care are addressed. Collaborating with experts across specialties is necessary as little is known about the pathology of this disease (1,2). There have only been two reported cases of complete resolution after pregnancy without intervention (1). However, the first line of therapy in gestational gigantomastia is medical treatment with bromocriptine. Bromocriptine has been noted to be safe in pregnancy and labeled as Food and Drug Administration Category B with no proven risk in humans; however, the resolution of gestational gigantomastia with bromocriptine alone is uncommon (8). While bromocriptine does not typically cause resolution of gestational gigantomastia, it can prevent disease progression for women identified earlier in their course (9,10). Many women are not identified until significant progression of disease as they are often dismissed by medical providers due to lack of knowledge surrounding this rare condition and moderate breast enlargement can be anticipated for women during pregnancy. The disease response to bromocriptine supports the prolactin/hormonal theory of gestational gigantomastia (1,4,5). However, bromocriptine was ineffective in our patient who presented at an advanced stage with her breast ulceration worsening and skin necrosis.
Surgery is the mainstay of management for patients with gestational gigantomastia, as medical therapy is often unsuccessful, and the progression of the disease leads to skin breakdown and can render patients immobile. Surgery is relatively safe during pregnancy, although there is an increased risk of preterm labor and delivery. The timing of surgery should be planned alongside multidisciplinary discussions with the obstetrics and gynecology team, with consideration given to avoiding early first-trimester interventions if possible to allow for optimal fetal lung maturation (11). In the first trimester surgical intervention should be delayed to the second trimester if possible. During the second trimester early delivery of the fetus is not optimal and therefore surgical intervention with mastectomy is typically ideal if definitive intervention is deemed necessary. During the third trimester risk versus benefit of early delivery versus surgical intervention should be discussed with a multidisciplinary team. If early delivery is planned, attempts should be made to augment fetal lung maturation with medications. Surgical intervention is typically a mastectomy. Breast reduction is not a durable treatment option as the breast tissue will continue to grow in the setting of pregnancy (1). In this case, a bilateral palliative mastectomy was performed in her second trimester with excellent patient recovery and a safe fetal outcome. Another component of the multidisciplinary management of gestational gigantomastia is the plastic surgery team, as flap coverage may be necessary if the patient does not have enough healthy tissue for closure post mastectomy. Fortunately, our patient did not require flap coverage based on her pattern of skin necrosis. Reconstruction is often delayed until the postpartum period to minimize operative time and potential postoperative complications most importantly implant related infection (1).
Conclusions
The limitation of our case report is that it lacks generalizability. Often, medical management of gestational gigantomastia is unsuccessful, and there is hesitancy to proceed with surgery in the setting of a viable pregnancy. However, surgical intervention or delivery are essential for definitive management of gestational gigantomastia to prevent secondary complications such as sepsis due to skin ulceration and necrosis and our successful case elucidates this treatment approach. Further studies should explore the benefits of surgery to medical management and possibly providing a standard treatment recommendation or guideline for this breast disease. All interventions should be discussed and made in the setting of a multidisciplinary team that includes surgery, anesthesia, plastic surgery, obstetrics and gynecology, and pathology to ensure optimal maternal and fetal outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-23-13/rc
Peer Review File: Available at https://asj.amegroups.com/article/view/10.21037/asj-23-13/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-23-13/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mangla M, Singla D. Gestational Gigantomastia: A Systematic Review of Case Reports. J Midlife Health 2017;8:40-4. [Crossref] [PubMed]
- Maluf I Junior, da Silva Freitas R, Budel VM, et al. Gestational gigantomastia: how to address this clinical situation. Brazilian J Plast Sugery 2015;30:134-7.
- Fletcher MB, Corsini LM, Meyer MD, et al. Gestational gigantomastia: A case report and brief review of the literature. JAAD Case Rep 2020;6:1159-61. [Crossref] [PubMed]
- Rezai S, Nakagawa JT, Tedesco J, et al. Gestational Gigantomastia Complicating Pregnancy: A Case Report and Review of the Literature. Case Rep Obstet Gynecol 2015;2015:892369. [Crossref] [PubMed]
- Türkan H, Gökgöz MŞ, Taşdelen İ, et al. Gestational Gigantomastia. J Breast Health 2016;12:86-7. [Crossref] [PubMed]
- Neblett C, Venugopal R, Johnson M, et al. Gestational gigantomastia-a rare entity complicated by life-threatening haemorrhage. J Surg Case Rep 2021;2021:rjab050.
- Rakislova N, Lovane L, Fernandes F, et al. Gestational gigantomastia with fatal outcome. Autops Case Rep 2020;10:e2020213. [Crossref] [PubMed]
- Ecanow NS, Chichura AM, Kopkash K, et al. Gestational gigantomastia complicated by breast infarctive necrosis in the setting of COVID-19 infection: a case report. Ann Breast Surg 2023;7:30. [Crossref]
- Moussaoui KE, Ansari AC, Baidada A, et al. Conservative management of gestational gigantomastia: A case report. J Gynecol Res Obstet 2021;7:024-027.
- Dharini Venkataram T, Raghuprakash S. Gestational gigantomastia with spontaneous resolution in an Indian woman. BMJ Case Rep 2018;2018:bcr2017224009. [Crossref] [PubMed]
- Qin F, Si L, Zhang H, et al. Management of gestational gigantomastia with breast reconstruction after mastectomy: case report and literature review. J Int Med Res 2020;48:300060520920463. [Crossref] [PubMed]
Cite this article as: Okere UC, Margenthaler JA, Vanko S, Kennard K. A multi-disciplinary approach to gestational gigantomastia management: a case report. AME Surg J 2023;3:47.


