
Internal acoustic meatus arteriovenous malformation: a rare illustrative case report and literature review
Highlight box
Key findings
• The internal acoustic meatus may be considered an eloquent location. This complex region has been neglected by existing classification systems. Embolization of the anterior inferior cerebellar artery and therefore the labyrinthine artery may result in sensorineural hearing loss.
What is known and what is new?
• It is known that posterior fossa arteriovenous malformations (AVMs), despite their rarity, carry a high rate of rupture and the potential to cause significant morbidity given the proximity of eloquent structures.
• This is the first report of an AVM of the internal acoustic meatus successfully treated with both endovascular and surgical means.
What is the implication, and what should change now?
• Both endovascular and surgical approaches are likely required to obliterate AVMs in this rare location.
Introduction
Background
Arteriovenous malformations (AVMs) are embryological high flow complexes consisting of feeding arteries connected by a nidus to draining veins in the absence of any intervening normal brain (1). ApSimon et al. published a large series of 240 AVMs and found that 94.9% were located in the supratentorial compartment, with the remainder being in the mesencephalon or cerebellum (2). Graf et al. presented their series of 134 patients with ruptured cerebral AVMs and only 11 were located within the posterior fossa (3).
Rationale and knowledge gap
An AVM of the internal acoustic meatus is therefore exceedingly rare, and this entity was neglected in the original Spetzler-Martin classification as an eloquent location despite housing critical neurovascular structures (4).
Objective
We present the first case of a unique supplied internal acoustic meatus AVM requiring multi-modality treatment in accordance with the CARE reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-23-39/rc). The role of both endovascular and surgical management is discussed.
Case presentation
A 46-year-old right handed female was found to have a left side AVM of the internal acoustic meatus on workup for nasal polyps. She reported hearing a ‘whooshing’ sound in her left ear for 5 years associated with intermittent pulsatile tinnitus. She experienced mild vestibular disequilibrium and feeling off balance at times though denied any hearing loss. Her past medical history was significant only for previous renal calculi and a cholecystectomy. She did not take any regular medications and was a non-smoker living at home.
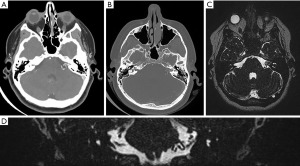
On examination, she was neurologically intact with a Glasgow Coma Scale (GCS) of 15. Unterberger’s test was negative. Preoperative pure tone audiometry showed normal hearing and speech discrimination in both ears with type A tympanograms consistent with normal middle ear function. Computed tomography (CT) of the brain demonstrated no significant widening of the left internal acoustic meatus compared to the contralateral side (Figure 1). CT angiography and magnetic resonance imaging (MRI) also revealed a complex 20 mm × 9 mm × 7 mm nidus of blood vessels in the left intracanalicular portion of the internal acoustic meatus extending into the cerebellopontine angle (Figure 1). There was a meatal loop arising from the anterior inferior cerebellar artery (AICA).
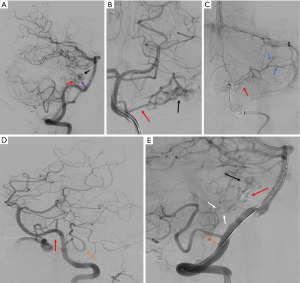
Cerebral digital subtraction angiography (DSA) confirmed the presence of a left internal acoustic meatus AVM with primary feeders from the left AICA and left posterior inferior cerebellar artery (PICA). Venous outflow was via cerebellar veins draining to the left sigmoid sinus (Figure 2). Given the majority of the arterial supply appeared to be from the endovascularly accessible single trunk AICA, this artery was cannulated and the feeding branch was preoperatively coiled. The left PICA was also cannulated but multiple small perforators were seen to be supplying the AVM and these were deemed too high risk to occlude. Post-embolization angiography on the 29th August 2022 demonstrated significantly reduced flow through AVM but some residual supply arising from branches of the teloveloteonsillar segment of the left PICA (Figure 2). Immediately following the endovascular embolization the patient developed a new complete left sided sensorineural hearing loss likely due to involvement of the labyrinthine artery during the procedure.
In order to definitively obliterate the arteriovenous malformation, she proceeded to undergo a left sided retrosigmoid craniotomy in the supine position on the 30th August 2022. The retrosigmoid approach was selected given it provided the most direct surgical access to the AVM which was located within the porus acousticus and medial internal acoustic meatus. Neuromonitoring of the 7th–12th cranial nerves was utilized. After arachnoid opening and release of cerebrospinal fluid from the cisterna magna, the AICA was easily identified due to the presence of the coil mass. A large vascular complex was identified surrounding and intermingled within the facial nerve with entangled loops of arterialised draining veins. Intraoperative indocyanine green was utilised to identify the arterial feeders which were sequentially coagulated and divided until the nidus was confirmed to no longer fill. The facial nerve remained intact but required 0.2 mA to stimulate compared to the initial 0.05 mA. An otologist was present for the surgery in case the posterior wall of the porus acousticus required removal, however this was not performed as it was felt the AVM had no further flow and any further manipulation of the nerve would likely cause a permanent facial palsy.
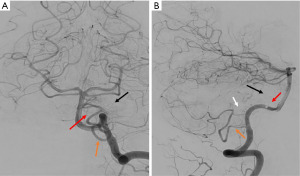
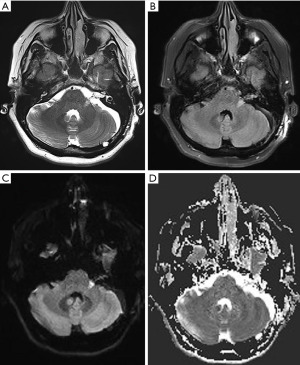
Post-operative cerebral angiography demonstrated successful complete obliteration of the AVM (Figure 3). Diffusion-weight imaging sequence revealed a small acute infarct of the left middle cerebellar peduncle likely related to endovascular embolization (Figure 4). On 3-month follow-up, she had persistent left sided sensorineural hearing loss but an improving House-Brackmann 3 palsy. She was reviewed by speech pathology both as an inpatient and outpatient given her hearing loss.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Posterior fossa AVMs are uncommon and estimated to constitute only 5–12% of all intracranial AVMs, but are more likely to present with haemorrhage and result in a poorer prognosis (5,6). Despite their rarity, these are important entities given their close proximity to deep eloquent structures (6). Indeed, Khaw et al. determined that the infratentorial location of these AVMs was itself an independent risk factor for haemorrhage [odds ratio (OR) 1.99, 95% confidence interval (CI): 1.07–3.69, P=0.03] (6). Haemorrhage of an AVM within the internal acoustic meatus, a cerebrospinal fluid containing cavity housing critical neurovascular structures, carries significant morbidity (7).
A comprehensive literature review of the MEDLINE, Embase, Google Scholar and Cochrane databases found only eight histopathologically proven cases of AVMs located within the internal acoustic meatus (Table 1) (7-14). The majority of these cases were reported during an era where archaic means such as X-rays to examine for widening of the internal acoustic meatus or gas CT cisternography to examine for outlined lesions were used, and only intra-operatively was an AVM conclusively confirmed (8). None of these historical cases utilized dual modality treatment. We present the first case of an unruptured AVM of the internal acoustic meatus supplied by branches of the AICA and PICA successfully treated with both endovascular and surgical treatment.
Table 1
| Author, year | Age (years), sex | Symptoms | Laterality | Arterial feeders | Venous drainage | Nidus size (mm) | Treatment and exclusion status | Clinical outcome |
|---|---|---|---|---|---|---|---|---|
| Linskey, 1991 (7) | 39, M | SNHL (deaf), vertigo, hemifacial spasm | Right | NR | NR | NR | ST, NR | Postoperative facial palsy improving |
| Sundaresan, 1976 (8) | 26, M | SNHL, hemifacial spasm, tinnitus | Right | NR | NR | NR | ST, NR | Facial palsy, SNHL |
| Bird, 1985 (9) | 44, M | SNHL, facial palsy | Left | AICA | NR | NR | ST, NR | Unchanged |
| Lo, 1986 (10) | NR | Facial palsy | Left | NR | NR | 6×4×4 | ST, NR | NR |
| Mahran, 1991 (11) | 50, F | SNHL, tinnitus, vertigo facial palsy | Right | AICA | Lateral bulbopontine vein | NR | ST, excluded | SNHL complete, tinnitus unchanged, vertigo improved, facial palsy improved |
| Patel, 2011 (12) | 50, M | Vertigo | Left | AICA, labyrinthine artery, transosseous and dural supply | Superior petrosal sinus, retrograde flow to lateral mesencephalic vein | 16×10 | ET, excluded | SNHL new post, Low pitched tinnitus, normal facial function |
| Ng, 2016 (13) | 53, F | SNHL, hemifacial hypoaesthesiae, facial palsy | Right | NR | NR | 11×10×12 | ST, excluded | Hemifacial hypoaesthesiae resolved, facial palsy improved, |
| Sulieman, 2018 (14) | 55, M | Asymptomatic | Right | NR | NR | NR | NR | NR |
| Present case | 46, F | Mild vestibular dysequilibrium | Left | AICA, PICA | Cerebellar veins into sigmoid sinus | 20×9×7 | ET and ST, excluded | House-Brackmann 4 facial palsy, sensorineural hearing loss |
M, male; F, female; SNHL, sensorineural hearing loss; NR, not reported; ST, surgical treatment; AICA, anterior inferior cerebellar artery; ET, endovascular treatment; PICA, posterior inferior cerebellar artery.
This unusual case poses three unique treatment challenges which are not readily addressed by conventional classification schemes. Firstly, the internal acoustic meatus is a complex and highly eloquent anatomical space. Martin et al. dissected both cerebellopontine angles in 25 adult cadavers and found that the AICA was a single trunk in the majority of cases (72%), occasionally duplicate (26%) and rarely triplicate (2%) (15). After its origin from the basilar artery, the AICA passes posteriorly, inferiorly and laterally across the pons (16). From here, the relationship to the cranial nerves has proven highly variable with the AICA usually travelling in between the facial/nervus intermedius nerves and the vestibulocochlear nerves (66%), but also sometimes travelling posterior (12%) or even inferior to the nerve complex (10%) (16). This highlights the importance of understanding individual patient anatomy by obtaining high resolution preoperative imaging with a combination of MRI and catheter angiography. In our case, the AVM nidus was entangled around and within the facial nerve itself with multiple adherent loops needing to be dissected free, though ultimately several small branches could not be dissected from the nerve and were left in situ. This was further complicated by the narrow window in which to work given this vascular malformation did not cause expansion of the cerebellopontine angle as is typically the case with tumours. Lack of expansion of the internal acoustic meatus, which again typifies more commonly found lesions in this area such as acoustic neuromas, was not a concern as we did not drill out the meatus for distal access in this case.
This case represents a clinical dilemma not easily resolved using the current paradigm of AVM treatment. The landmark Spetzler-Martin classification was developed and validated based upon 100 consecutive retrospectively identified AVMs (17). The surgical risk of these lesions was stratified based upon three factors: size of the nidus being less than 3 cm (1 point), 3–6 cm (2 points) or >6 cm (3 points), location in either non-eloquent (0 points) or eloquent brain (1 point) and pattern of venous drainage being either superficial (0 points) or deep (1 point) (17). Spetzler et al. defined deep eloquent areas as being the thalamus, hypothalamus, brainstem and deep cerebellar nuclei (17). There is no mention of the internal acoustic in the original landmark publication (17). However, the ethos remains that the immediate risk of operative intervention to resect an AVM must be weighed up against the estimated up to 11.6% higher rate of posterior AVM rupture per year and the attendant 50% risk of neurological deficit associated with this (4,17). Fults et al. remind us that posterior fossa AVMs are associated with a much poorer prognosis (18,19).
Further complicating the treatment decision, we encountered a relatively young otherwise healthy patient with completely preserved hearing. Complete exclusion of the AVM in this location without sacrificing the delicate labyrinthine artery seemed unlikely. Indeed, 5 of the 8 previously documented patients with AVMs in this location presented with sensorineural hearing loss (Table 1) (7-9,11,13). Of the other three cases, Patel et al. reported new hearing loss in their 50-year-old patient with a left sided AVM also supplied by the AICA which was treated solely by endovascular means, whilst the authors of the other two cases did not comment on hearing status (10,12,14). Mangham et al. warned clinicians of the significance of the AICA given in their series of intratemporal tumours the vascular lesions were more likely to cause hearing loss than acoustic neuromas of comparable size (20). It was Lo et al. who supported this sentiment also found that schwannomas tended to be larger than vascular tumours at presentation yet caused a similar degree neurological deficit (10).
Moreover, Lawton et al. recognized that there were also other factors which could complicate surgical resection such as diffuseness of the nidus on angiography and more or less arteriovenous connections resulting in higher flow (21). This was the rationale behind developing a supplementary AVM grading scheme for clinicians (21). We also took into consideration the fact that this AVM had no associated aneurysms, defined as dilatations of the lumen twice the width of the arterial vessel, which have been identified as a negative predictive outcome measure by Khaw et al. (6). On the other hand, smaller AVMs are possibly more likely to rupture than larger lesions at 1 year (10% versus 0%) and 5 years (52% versus 10%) (17).
This is amidst controversial evidence that medical management for unruptured AVMs may be superior to surgical treatment. The ARUBA randomized controlled trial, consisting of 233 patients with unruptured and never treated AVMs who were followed up for a mean of 33 months, found a decreased risk of death or stroke in the group who underwent surveillance rather than surgical management (22). Indeed, the hazard ratio of 0.27 (95% CI: 0.14–0.54) was so convincing that the trial was prematurely ceased (22). However, the trial has attracted many criticisms ranging from the fact that the majority of the AVMs in both the medical and surgical arms were Spetzler-Martin grade 1 and 2 (55% and 67%), there was a low rate of flow-related aneurysms (19% and 13%) and the majority only had superficial rather than deep drainage (63% and 69%) (22). Grasso et al. noted that these factors favour medical management (23). Although our case with a small 20 mm nidus resembled most of the AVMs in the surveillance arm (55%) and intervention arm (68%) being less than 30 mm in diameter, the location in the posterior fossa was a minority (5% and 7%) (22). Rutledge et al. also noted the follow-up was relatively short with Lawton et al. arguing surgery for low grade Spetzler-Martin lesions is still feasible (24,25).
This argument has been countered by Al-Shahi Salman et al. who examined a Scottish cohort with a longer 12-year follow-up of patients with unruptured AVMs (26). This observational study determined that there was less chance of achieving the primary outcome (occurrence of handicap) with conservative management rather than surgery (OR 0.59, 95% CI: 0.35–0.99) (26). Furthermore, the risk of stroke or death as a similar outcome measure to the ARUBA trial over 12 years again favoured surveillance over surgery (OR 0.37, 95% CI: 0.19–0.72) (26).
However, the authors did acknowledge the possibility of selection bias given those who underwent surgical intervention were younger, more likely to have presented with a seizure and also has smaller AVM nidus sizes (26). This trial also bears only minimal relevance to our rare case given only 4% of AVMs in the conservative arm and 3% in the surgical arm were located in the posterior fossa, with all of these being either in the brainstem or cerebellum (26). It is evident that larger trials with longer-term follow-up are still required in this domain.
The final modality of treatment to be considered in this case is stereotactic radiosurgery (SRS) with the primary goal being to obliterate the AVM nidus (27). Bowden et al. delivered SRS to 64 patients with cerebellar AVMs at a single institution and reported total obliteration rates as confirmed on MRI or angiography of 69% at 4 years, and 76% at 5 and 10 years (28). Interestingly, obliteration was more likely in those patients who had not undergone prior embolization (28). In their series, 6% of patients sustained a haemorrhage whilst awaiting complete obliteration which was countered by 1.5% of patients sustaining a permanent neurological deficit (28). Kelly et al. also argued that if angiographic obliteration did not occur by 3 years, then further treatment should be considered (29). SRS was considered as an option for our patient with the possibility of hearing preservation, but surgery was favoured given her significant symptoms of vestibular disequilibrium which were more likely to resolve with surgical resection rather than SRS (29).
Limitations of our study include the fact this is a rare tumour and reliant upon our single case experience. Future studies should include large randomized trials. Additionally, existing AVM classification systems fail to recognize the internal auditory meatus (IAM) as either an eloquent or non-eloquent location and therefore did not provide relevant evidence-based management suggestions.
Conclusions
The internal acoustic meatus should be considered an eloquent location when considering the treatment of AVMs. Given the known high rate of haemorrhage of posterior fossa AVMs, definitive management should be considered in younger patients. Careful evaluation of accessibility of arterial feeders and venous outflow by preoperative cerebral angiography is required. Multi-disciplinary discussion between neuro-radiologists and neurosurgeons is ultimately required, but both endovascular and surgical approaches are likely required to obliterate AVMs in this rare location.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-23-39/rc
Peer Review File: Available at https://asj.amegroups.com/article/view/10.21037/asj-23-39/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-23-39/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Steiger HJ. Recent progress understanding pathophysiology and genesis of brain AVM-a narrative review. Neurosurg Rev 2021;44:3165-75. [Crossref] [PubMed]
- ApSimon HT. A population-based study of brain arteriovenous malformation: long-term treatment outcomes. Stroke 2002;33:2794-800. [Crossref] [PubMed]
- Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg 1983;58:331-7. [Crossref] [PubMed]
- Arnaout OM, Gross BA, Eddleman CS, et al. Posterior fossa arteriovenous malformations. Neurosurg Focus 2009;26:E12. [Crossref] [PubMed]
- da Costa L, Thines L, Dehdashti AR, et al. Management and clinical outcome of posterior fossa arteriovenous malformations: report on a single-centre 15-year experience. J Neurol Neurosurg Psychiatry 2009;80:376-9. [Crossref] [PubMed]
- Khaw AV, Mohr JP, Sciacca RR, et al. Association of infratentorial brain arteriovenous malformations with hemorrhage at initial presentation. Stroke 2004;35:660-3. [Crossref] [PubMed]
- Linskey ME, Jannetta PJ, Martinez AJ. A vascular malformation mimicking an intracanalicular acoustic neurilemoma. Case report. J Neurosurg 1991;74:516-9. [Crossref] [PubMed]
- Sundaresan N, Eller T, Ciric I. Hemangiomas of the internal auditory canal. Surg Neurol 1976;6:119-21. [PubMed]
- Bird CR, Drayer BP, Yeates AE. Gas CT cisternography of an intracanalicular vascular malformation. AJNR Am J Neuroradiol 1985;6:969-70. [PubMed]
- Lo WW, Horn KL, Carberry JN, et al. Intratemporal vascular tumors: evaluation with CT. Radiology 1986;159:181-5. [Crossref] [PubMed]
- Mahran A, Samii M, Penkert G, et al. Vascular lesions of the internal auditory canal. Skull Base Surg 1991;1:78-84. [Crossref] [PubMed]
- Patel PN, Connor S, Brew S, et al. An arteriovenous malformation within the internal acoustic meatus and cerebellopontine angle cistern. J Laryngol Otol 2011;125:1275-8. [Crossref] [PubMed]
- Ng R, Rahman RA, Bakar AA, Abdullah A. A rare case of arteriovenous malformation in the internal acoustic meatus. RMJ 2016;41:250-2.
- Sulieman N, Basura G. Rare presentation of an arteriovenous malformation within the internal auditory canal. Otolaryngol Case Rep 2018;6:10-3. [Crossref]
- Martin RG, Grant JL, Peace D, et al. Microsurgical relationships of the anterior inferior cerebellar artery and the facial-vestibulocochlear nerve complex. Neurosurgery 1980;6:483-507. [Crossref] [PubMed]
- Alonso F, Kassem MW, Iwanaga J, et al. Anterior Inferior Cerebellar Arteries Juxtaposed with the Internal Acoustic Meatus and Their Relationship to the Cranial Nerve VII/VIII Complex. Cureus 2017;9:e1570. [Crossref] [PubMed]
- Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg 1986;65:476-83. [Crossref] [PubMed]
- Fults D, Kelly DL Jr. Natural history of arteriovenous malformations of the brain: a clinical study. Neurosurgery 1984;15:658-62. [Crossref] [PubMed]
- Matsumura H, Makita Y, Someda K, et al. Arteriovenous malformations in the posterior fossa. J Neurosurg 1977;47:50-6. [Crossref] [PubMed]
- Mangham CA, Carberry JN, Brackmann DE. Management of intratemporal vascular tumors. Laryngoscope 1981;91:867-76. [Crossref] [PubMed]
- Lawton MT, Kim H, McCulloch CE, et al. A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery 2010;66:702-13; discussion 713. [Crossref] [PubMed]
- Mohr JP, Overbey JR, Hartmann A, et al. Medical management with interventional therapy versus medical management alone for unruptured brain arteriovenous malformations (ARUBA): final follow-up of a multicentre, non-blinded, randomised controlled trial. Lancet Neurol 2020;19:573-81. [Crossref] [PubMed]
- Grasso G. The ARUBA study: what is the evidence? World Neurosurg 2014;82:e576. [Crossref] [PubMed]
- Rutledge WC, Abla AA, Nelson J, et al. Treatment and outcomes of ARUBA-eligible patients with unruptured brain arteriovenous malformations at a single institution. Neurosurg Focus 2014;37:E8. [Crossref] [PubMed]
- Lawton MT, Abla AA. Management of brain arteriovenous malformations. Lancet 2014;383:1634-5. [Crossref] [PubMed]
- Al-Shahi Salman R, White PM, Counsell CE, et al. Outcome after conservative management or intervention for unruptured brain arteriovenous malformations. JAMA 2014;311:1661-9. [Crossref] [PubMed]
- Pohjola A, Lehto H, Hafez A, et al. Arteriovenous Malformations of the Posterior Fossa: Focus on Surgically Treated Patients Presenting with Hemorrhage. World Neurosurg 2018;116:e934-43. [Crossref] [PubMed]
- Bowden G, Kano H, Tonetti D, et al. Stereotactic radiosurgery for arteriovenous malformations of the cerebellum. J Neurosurg 2014;120:583-90. [Crossref] [PubMed]
- Kelly ME, Guzman R, Sinclair J, et al. Multimodality treatment of posterior fossa arteriovenous malformations. J Neurosurg 2008;108:1152-61. [Crossref] [PubMed]
Cite this article as: Kweh BTS, Gonzalvo A, Asadi H, Maingard J, Russell J. Internal acoustic meatus arteriovenous malformation: a rare illustrative case report and literature review. AME Surg J 2023;3:51.


