Patient reported outcomes and aesthetic assessment following chest wall perforator flap partial volume replacement for primary breast cancer
Highlight box
Key findings
• This study confirms that we can achieve equivalent patient reported outcome measures (PROMs) following breast conservation as for with standard breast conservation surgery; BREAST-Q ‘satisfaction with breasts’ mean of 67.6, standard deviation (SD): ±18.8 (range, 39–100).
• There is minimal back morbidity associated with laterally placed chest wall perforator flaps (CWPFs) with patients’ reporting a mean ‘satisfaction with back’ score of 80.1, SD: ±19.9 (range, 40–100).
What is known and what is new?
• The use of CWPFs has become an increasingly popular oncoplastic technique to provide volume replacement after oncoplastic breast surgery with favourable patient reported outcomes.
• This is the first study to utilise 3D imaging to assess aesthetic outcome in this cohort of patients and we report a good aesthetic result [global outcome of 3.4, SD: ±1.1 (range, 1–5) based on the Delphi score].
What is the implication, and what should change now?
• CWPF can be offered to patients for volume replacement highlighting favourable patient and aesthetic outcomes and a low risk of shoulder and back dysfunction (with laterally sited flaps).
• Work needs to be done to create a validated assessment tool to measure post-operative outcomes for patients having CWPF for volume replacement as it gains popularity.
Introduction
Background
Oncoplastic techniques for breast conserving surgery (BCS) broadly comprise volume displacement and volume replacement (1). The latter term ‘volume replacement’ was first described by Raja et al. in 1997 (2). In small to medium sized breasts (up to C or D cup size), volume replacement techniques could be utilised, especially for relatively large tumours in the absence of parenchymal ptosis. These require replacement of tissue into the tumour excision defect from a regional or distant site.
The workhorse of volume replacement for breast reconstruction has been the latissimus dorsi (LD) flap for many decades. However, sacrifice of one of the largest muscles of the torso does lead to morbidity including impaired functional outcome (3). This, along with a better understanding of regional distribution of perforator vessels supplying the lateral and inferior chest wall has led to the evolution of muscle-sparing (fascio-adipo-cutaneous) pedicled perforator flaps (4). A lateral thoracic artery perforator (LTAP) flap can be used exclusively or in combination with a lateral intercostal artery perforator (LICAP) flap to reconstruct lateral, central, lower outer or upper medial situated breast excision defects. The anterior intercostal artery perforator (AICAP) or medial intercostal artery perforator (MICAP)-based flaps can be used for partial breast reconstruction for lower central and lower or upper inner quadrant defects respectively. Figures 1-3 demonstrate the utility of both lateral and anterior chest wall perforator flap (CWPF) in patients who were included in this study.
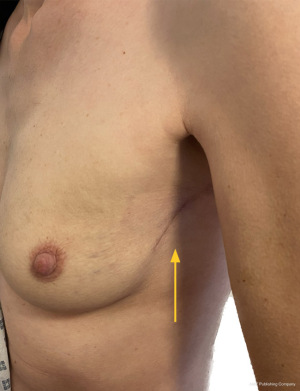
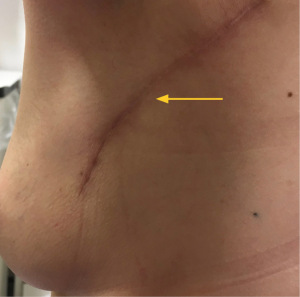
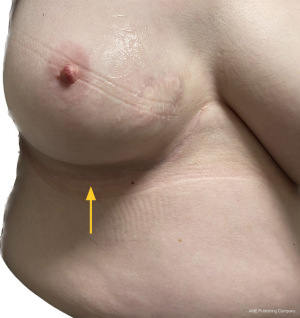
The role of breast conserving surgery can now be extended with techniques such as local perforator flaps. Local flaps are particularly useful for partial breast volume replacement due to the relative simplicity of the surgical procedure, low morbidity and short post-operative recovery. A potential advantage over displacement techniques, symmetrisation of the contra-lateral breast is usually not required in cases for volume replacement. It therefore has the benefit of potentially being a one-stage, unilateral procedure reducing the need for mastectomy and associated recovery and complications associated with immediate reconstruction (5).
Rationale and knowledge gap
Although described more than a decade ago by Hamdi et al. (4), CWPFs were not initially adopted widely in the United Kingdom (UK). Now, several UK centres perform CWPFs for volume replacement and studies by McCulley et al. (6) and more recently Quinn et al. (7) have reinforced the effectiveness of CWPF. Initial reported of patient and aesthetic outcomes are positive (8-14), however, there is no validated patient reported outcome measures (PROMs) instrument specifically for partial breast reconstruction with CWPF, which spares the LD muscle.
PROMs are instruments to measure any aspect of a patient’s health status and satisfaction with overall aesthetic outcome.
The BREAST-Q is a multidimensional questionnaire-based tool that assesses PROMs following breast treatment. It is a rigorously developed patient-reported outcome measure for use in cosmetic and reconstructive breast surgery and clinical practice (15). The BREAST-Q was developed quantitatively and qualitatively to measure patients’ perceptions before and after breast surgery. It does this by examining quality of life domains (psychosocial well-being, physical well-being, sexual well-being) and satisfaction domains (satisfaction with breasts, satisfaction with outcome, satisfaction with care) (16,17).
Several studies have utilised BREAST-Q to assess PROMs in patients following BCS. Dahlbäck et al. (18) report a series of over 300 women with a median ‘satisfaction with breasts’ Q-score of 66 after BCS, and psychosocial wellbeing Q-score of 82. O’Connell et al. corroborates these findings in a series of 200 women 1–6 years after BCS with a median Q-score for ‘satisfaction with breasts’ of 68, and 82 for psychosocial wellbeing (19). Whilst they included volume displacement techniques, volume replacement was omitted. Currently, there is no validated PROMs instrument specifically for partial breast reconstruction with CWPF, which spares the LD muscle.
Aesthetic outcome from breast cancer surgery has a well-documented influence on patients’ psychosocial wellbeing and quality of life (20). Panel assessment is the most widely accepted technique to measure aesthetic outcome in breast surgery, however, has inherent bias, is costly, time consuming, and unstandardised. The most widely adopted scale for use within breast conserving therapy (BCT) is the Harvard cosmesis scale, developed by Harris et al. in the 1970s (21). It reports symmetry between breasts using a 4-point Likert scale from 1, poor to 4, excellent (Table S1).
3D-surface imaging (SI) has the potential to overcome the limitations of alternative methods of evaluating aesthetics. It is simple to use and provides multiple views from one capture including the cranial and caudal views which help visualise projection and the Infra Mammary Fold (IMF). It delivers linear mammometrics in addition to volume and surface symmetry calculations. The images can be captured using Vectra® (Canfield, USA). Vectra® is a 3D imaging system that combines total body photography in ultra-high resolution 3D with software that enables the images to be reconstructed and viewed as a 3D graphic. Women are positioned with their hands on their hips with their elbows behind the mid-axillary line to optimise visualisation of the lateral aspect of the breast. Images are taken at the end-inspiratory pause during quiet breathing.
3D-SI is purported to be a precise and accurate way to measure volume and symmetry (22-24). The Delphi-derived scoring system was designed as a scale against which an objective measure of aesthetic outcome for breast reconstruction can be developed using measures derived from 3D-SI (see Table S2). It consists of a scale of 1–5 and six domains are assessed: shape; volume; nipple position; position of breast mound; symmetry; global appearance.
Objective
The primary aim was to determine PROMs after CWPFs for volume replacement in BCS and compare this to existing PROMs data on breast conservation.
Secondary aims
- Panel assessment after CWPFs on 2D photography and 3D surface imaging;
- Determine whether CWPF has a comparable subjective assessment of the severity of back morbidity compared with LD reconstruction in the literature;
- Determine which patient, tumour and treatment-related factors are associated with the above.
We present this article in accordance with the STROBE reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-23-21/rc).
Methods
Patient population
This is a prospective cohort study of female patients who have undergone volume replacement with CWPF reconstruction following breast surgery at The Royal Marsden Hospital in the UK over the last 5 years.
Inclusion criteria
- Female patients who have had breast conserving surgery with partial volume replacement using CWPF for primary breast cancer at The Royal Marsden Hospital Trust in the last 5 years were eligible.
Exclusion criteria
- Prior breast surgery to either breast or concomitant surgery to contralateral breast.
- Patient deceased or lost to follow up.
- Unable to answer the BREASTQ questionnaire (e.g., learning difficulties).
Tumour and operative details
All patients were operated on by consultant oncoplastic breast surgeons using a single stage approach. The perforator vessels were identified with use of a Doppler probe pre-operatively in clinic and on the morning of surgery. The estimated volume of defect and flap marking was performed with patient in lying and standing position. For AICAP and MICAP flaps the infra-mammary fold is marked. For exposure of the LTAP/LICAP donor site a small sand bag was placed beneath the ipsilateral paraspinal area to achieve a tilt and the arm board was raised to support the arm or the patients were positioned in a lateral position and turned to supine for flap placement. A single incision was used for the wide local excision and reconstruction in all cases. In some cases, the axilla was also accessed through the same incision. The flaps were de-epithelized prior to placing them in the resection cavity. Drains were used for the donor site on a discretionary basis. Patients were discharged on the same day and followed up in clinic 2 weeks later.
Patient demographics including age, body mass index (BMI) and co-morbidities reported in the pre-operative assessment were recorded. Tumour size, receptor status and specimen weight (based on the post-operative pathology reports) were also recorded on a prospectively maintained database.
Patient reported outcomes
An electronic BREAST-Q distributed via e-mail was sent and patients were also invited for medical 2D and 3D photography on site. Patients whose preference was to complete the survey on paper were sent a paper copy. The BREAST-Q modules utilised were the BCS, breast reconstruction and LD and included questions related to: psychosocial wellbeing; sexual wellbeing; satisfaction with breasts; physical wellbeing (chest and back); adverse effects of radiation. Patients were also asked how they received information prior to surgery, the pain, softness, and lumpiness of the treated and contralateral breast, the amount of help required with daily activities, and the overall results of surgery. The BREAST-Q data are transformed into scores ranging from 0–100 according to the guidelines provided by BREAST-Q with higher scores indicating more favourable outcomes. All responses were anonymised.
Panel assessments
Objective outcomes were measured using the Harvard cosmesis scale for 2D photography and the Delphi scoring system for 3D surface imaging (Tables S1,S2) (21,25). The scoring outcome of the Harvard cosmesis scale for 2D photography is defined as excellent [4], good [3], fair [2], and poor [1]. The scoring outcome for the Delphi score is defined as excellent [5], good [4], moderate [3], poor [2], very poor [1] in seven domains: shape; volume; nipple position; projection; position of breast mound; symmetry; global.
All members of the panel had participated in objective panel assessments in the past and had experience in the protocol as well as informed of the study design.
For the assessment, the panel of four consisted of:
- Two breast surgeons (one consultant, one senior fellow);
- One consultant clinical oncologist;
- One consultant plastic surgeon.
The panel were first presented with a poor outcome and an excellent outcome as an example of the lower and upper limits of scale. This was determined by an independent oncoplastic breast surgeon prior to the formal panel assessment. The panel had the opportunity to review and question the scoring systems ahead of the assessment. The assessment was performed virtually and marks were recorded independently and anonymously from the other panel members. The average of the scores across the panel was then calculated as the overall outcome score for that patient’s 2D or 3D image.
Univariate regression analysis was used to assess potential contribution of patient/clinico-pathological variables:
- Demographics: age and BMI at surgery (continuous), smoking status (non-, current or current within 6 weeks of surgery, dicotomized).
- Tumour-related: maximum tumour dimension (mm) and weight of specimen (g).
- Treatment-related: quadrant of resection/volume replacement, axillary surgery extent [nil vs. sentinel lymphadenectomy (SLND) vs. axillary lymphadenectomy (ALND)], re-excision of margins, post-operative complications, radiotherapy and radiotherapy boost as dicotomized variables.
Statistical analysis
Data was analysed using IBM SPSS advanced statistics (Statistical Package for Social Sciences), version 24 (SPSS Inc., Chicago, IL, USA).
Demographics are presented as descriptive statistics, and any quantitative variables presented as mean and standard deviation (SD) or median and interquartile range (IQR), as appropriate after testing for normality (Kolmogorov-Smirnov test). Qualitative data is presented as proportions and frequencies.
Scores for 2D and 3D-SI were obtained using a 4-point and 5-point Likert scale respectively and transformed into a 0–100 scale to perform univariate analyses by logistic regression against the BREAST-Q scores.
Two-sided univariate regression analyses were used to assess whether any independent variables affect patient reported outcome. A P value of less than P<0.1 was deemed statistically significant and would be entered into a multivariable model to identify any independent risk factors (as above).
The Wilcoxon Signed Rank Test was used to test the agreement of 2D and 3D panel assessment vs. patient related outcome assessed by the BREAST-Q questionnaire.
The primary endpoint was PROMs quantification using Breast-Q. Secondary endpoints were panel assessment on 2D photography and 3D surface imaging; Measurement by BREAST-Q the subjective assessment of the severity of back morbidity and analysis of patient, tumour and treatment-related factors associations with the above outcomes. Missing data from the database was recovered via the electronic patient records system.
The use of a prospectively maintained database to record patient and tumour characteristics minimised potential bias and confounders. Non-response bias was addressed with the use of a pre-notification e-mail containing information about the survey and the use of a personalised invite and a reminder.
Ethical considerations
The study was approved by the internal audit committee (Project Ref: BR2021_168) and further authorised via the University of East Anglia Ethical Committee. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all the patients.
Results
Patient and tumour characteristics
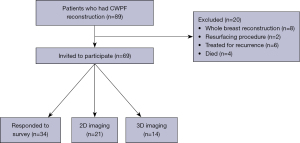
Seventy-three patients had CWPF for partial volume replacement in the context of breast conservation as part of treatment for primary breast cancer. Four patients had died from progressive metastatic disease at the time of PROMS assessment and were therefore excluded. Therefore 69 patients were invited to participate (Figure 4).
The response rate to the questionnaire was 53.6% (37/69) with the majority of patients choosing to provide their feedback via the online portal rather than written survey. Three patients did not provide an identifier and therefore could not be included in the univariate analysis.
Of our identifiable respondents (n=34) the median age was 55 (IQR, 34–63) years and BMI was 25.5 [IQR, 22.1–30.2] kg/m2. The mean time from breast surgery to participation in the study was 5.7 (SD: ±6.4, range, 1–40) months. The median tumour size was 45 (IQR, 35–65) mm with a median surgical specimen weight of 71 (IQR, 43–92) g. The patient, tumour and treatment characteristics of the respondents are summarised in Table 1. Of these patients, 25 had laterally situated perforator flaps [LTAP/LICAP/thoracodorsal artery perforator (TDAP)] and the other nine anteriorly based flaps (AICAP/MICAP). Most patients responded to the questionnaire at a time point after adjuvant radiotherapy (29/34) with the remaining five patients in the post-operative period pre-radiotherapy (5/34).
Table 1
| Characteristics | Values |
|---|---|
| Age (years) | 55 [34–63] |
| BMI (kg/m2) | 25.5 [22.1–30.2] |
| Current smokers | 2 |
| Ex-smokers | 8 |
| Radiotherapy | 29* |
| Adjuvant chemotherapy | 11 |
| Tumour size (mm) | 45 [35–65] |
| Specimen weight (g) | 71 [43–92] |
| Type of flap | |
| Anterior/inferior | |
| AICAP | 6 |
| MICAP | 3 |
| Lateral | |
| LICAP only | 7 |
| LTAP only | 7 |
| LICAP/LTAP combo | 10 |
| TDAP | 1 |
| Axillary surgery | |
| SLND only | 24 |
| ALND | 4 |
| None | 6 |
| Re-excision of margins | 3 |
| Involved margins <1 mm | 4 |
| Neuropathic pain | 1 |
| Wound complication | 1 |
Data are presented as median [IQR] or number. *, 1 of whom had boot to tumour bed; clear margin defined as ‘no ink on tumour’. BMI, body mass index; AICAP, anterior intercostal artery perforator; MICAP, medial intercostal artery perforator; LICAP, lateral intercostal artery perforator; LTAP, lateral thoracic artery perforator; TDAP, thoracodorsal artery perforator; SLND, sentinel lymphadenectomy; ALND, axillary lymphadenectomy; IQR, interquartile range.
Patient reported outcomes
The BREAST-Q scores are summarised in Table 2. All of the results were distributed non-parametrically, however, mean and SD are displayed to allow comparison with other published literature where the mean and SD have been stated. The nine patients who had anterior perforator flaps (AICAP/MICAP) did not contribute to the domains relating to back satisfaction. The highest scoring domains were ‘satisfaction with back’ and ‘satisfaction with information from surgeon’ scoring a median of 78 (IQR, 68–100) and 74 (IQR, 62–88) respectively. The lowest scoring domain was ‘sexual well-being’, with a median score of 39 (IQR, 26–64).
Table 2
| Domain | Median [IQR] | Mean ± SD [range] |
|---|---|---|
| Overall satisfaction with breast | 64 [53–83] | 67.6±18.8 [39–100] |
| Psycho-social well-being | 73 [58–88] | 72.4±19.5 [37–100] |
| Satisfaction with reconstruction | 64 [57–73] | 66.9±16.8 [37–100] |
| Physical well-being (chest) | 68 [60–81] | 68.1±21.2 [28–100] |
| Satisfaction with back | 78 [68–100] | 80.1±19.9 [40–100] |
| Physical well-being (shoulder/back) | 68 [49–80] | 66.4±20.1 [39–100] |
| Sexual well-being | 39 [26–64] | 46.2±24.3 [20–91] |
| Radiotherapy outcome | 45 [30–69] | 44.4±21.2 [33–78] |
| Satisfaction with information from surgeon | 74 [62–88] | 75.3±18.25 [25–100] |
IQR, interquartile range; SD, standard deviation.
Response rates varied within the domains ranging from 83.7% to 100% with the most poorly answered being sexual well-being (31/37 respondents).
Univariate analysis of patient reported outcomes based on variables
Of the 34 patients where tumour and patient characteristics were available a univariate analysis was used to identify clinico-pathological variables which were associated with outcomes. Across these variables there were no identifiable statistically significant (P>0.99) with patients reported outcomes (Table 3).
Table 3
| Variable | N | Correlation coefficient | P |
|---|---|---|---|
| BMI | 34 | −0.157 | 0.376 |
| Age | 34 | 0.047 | 0.793 |
| Specimen weight | 34 | −0.132 | 0.458 |
| Size of specimen | 34 | 0.011 | 0.951 |
| Smoking status | 0.773 | ||
| Ex-smoker | 2 | – | |
| Current smoker | 8 | – | |
| Type of chest wall perforator flap | 34 | – | 0.894 |
| Radiotherapy | 24 | – | 0.539 |
| Axillary surgery | |||
| SLND | 24 | – | 0.867 |
| ALND | 6 | – | 0.064 |
| Re-excision | – | – | >0.99 |
| Cancer location | – | – | 0.650 |
| Surgical complications | – | – | >0.99 |
BMI, body mass index; SLND, sentinel lymphadenectomy; ALND, axillary lymphadenectomy.
Aesthetic outcomes based on 2D photography
Twenty-one patients consented to have 2D photography for the panel assessment.
The scores were averaged from the individual panel scores with a mean of 2.7, SD: ±0.9 (range, 1–4). Four patients had sub-optimal results (<2), seven patients had fair results (2–3) and 10 patients had good or very good results (>3).
Aesthetic outcomes based on 3D photography
Fourteen patients consented to have 3D photography for the panel assessment.
Panel scores were averaged and a mean score ± SD were calculated (Table 4).
Table 4
| Assessment | Mean score ± SD |
|---|---|
| Shape | 3.3±1.1 |
| Volume | 3.6±0.97 |
| Nipple position | 3.25±1.4 |
| Projection | 3.5±1.0 |
| Position of breast mound | 3.4±1.2 |
| Symmetry | 3.25±1.1 |
| Global outcome | 3.4±1.1 |
SD, standard deviation.
The global outcome of the 14 patients was favourable, with two patients having an unsatisfactory outcome (<2), seven patients having a satisfactory outcome (2–4) and four patients having a good or excellent outcome (>4).
Comparison of 2D and 3D imaging
A total of 11 patients underwent panel assessment had both 2D and 3D surface imaging. A Wilcoxon Signed Rank Test was performed, P=0.032 demonstrating an overall statistically significant difference (P<0.05) between the 2D and 3D scoring favouring 3D-SI.
Comparison of panel assessment vs. PROMS
The Wilcoxon Signed Rank test was used to test the agreement of 2D and 3D-SI vs. patient related outcome assessed by the BREAST-Q questionnaire. This demonstrated a statistically non-significant difference (P=0.084 and P=0.272) respectively between the outcomes.
Discussion
Key findings
CWPFs are a significant addition to the oncoplastic breast surgery repertoire that can be offered to women to facilitate BCS without compromising cancer resection and in most patients, negates the need for mastectomy.
This study shows that CWPFs offer a good option for partial breast reconstruction with favourable patient-reported outcomes and minimal morbidity. Our series reports a low complication rate in line with the published evidence (11,12,14) with no patients in our cohort requiring invasive intervention for management of complications.
Strengths and limitations
There are several limitations to our study. As a feasibility study, small sample size and the relatively short time to follow-up clearly limit our analysis. Our study population represents a convenience sample of patients willing to take part in the BREAST-Q survey with attendant selection bias. This is a single-centre experience at a tertiary centre and although results are consistent with existing published outcomes it may not be generalizable to a broader population at present. As a snapshot of PROMs and photography following treatment, we lack baseline data as well as longitudinal pre- and post-radiotherapy data. This limits the ability to understand longitudinal changes in patient satisfaction experienced during the process of treatment and creates an inherent recall bias. Our inability to identify any statistically significant predictive variables that may have influenced PROMs in this patient cohort may be due to small sample size and lack of power. As increasing numbers of patients undergo volume replacement by CWPF the knowledge gap in the short and longer-term outcomes needs to be addressed.
Comparison with similar researches
To our knowledge this is the first study reporting BREAST-Q results with the addition of aesthetic outcome panel assessment utilising both 2D and 3D imaging in patients who have undergone breast conservation with CWPF for partial volume replacement. The UK’s Association of Breast Surgeon’s and the British Association of Plastic and Reconstructive and Aesthetic Surgery recognise the use of PROMs as an end-point in studies and a tool for quality control and it is widely accepted that the aesthetic and functional outcomes after breast cancer surgery correlate with higher quality of life (26).
A mean score of 67 for ‘satisfaction with breasts’ in our BCS patients is comparable to previously published studies that have assessed patients following BCS utilising BREAST-Q. Dahlbäck et al. (18) report a series of over 300 women with a median ‘satisfaction with breasts’ Q-score of 66 after BCS, and psychosocial wellbeing Q-score of 82. O’Connell et al. corroborates these findings in a series of 200 women 1–6 years after BCS with a median Q-score for ‘satisfaction with breasts’ of 68, and 82 for psychosocial wellbeing (19).
The panel assessment scores for 2D imaging had an average Harvard score of 2.7, SD: ±0.9 (range, 1–4) which is comparable to score for patients who have undergone standard breast conserving surgery. In a study by Godden et al. looking at the utility of 3D-SI a panel assessment of 190 patients undergoing BCT utilised the same score on 2D images as a benchmark and reported a mean of 2.87 (27).
Regarding assessment of physical well-being of the shoulder and back our cohort of patients scored favourably with an overall mean score of 66.4 compared to 64 from a study conducted by Kim et al. (28) and 40.63 from a study by Gao et al. (29) both of whom assessed this domain in patients undergoing LD flap reconstruction. The satisfaction with back score in our cohort was 80.1 compared to 52.5 in Gao et al. (29). The complication rate is also similar to that reported in the literature with a similar operative time. This supports the use of the (muscle-sparing) LICAP flap as an alternative to traditional LD mini-flap reconstruction with a trend towards more favourable patient reported outcomes. We did not objectively measure the impact of axillary surgery performed and whether this was a predictive factor for poorer outcome in this domain. In the future, with a larger cohort of patients we would like to assess the impact of the type of axillary surgery on the overall satisfaction with back and shoulder outcomes.
Explanation of findings
We were able to demonstrate the utility of 3D imaging in the assessment of patients post breast reconstruction and lends support to its utilisation more commonly to determine aesthetic outcome where feasible. We hypothesise that the higher correlation between PROMS and 3D panel assessment outcomes demonstrates that 3D imaging may give a better perception of results.
Implications and actions needed
To further develop this study, we would aim to use 3D imaging and volumetric assessment to objectively measure volume asymmetry between the two breasts and to also map the areas of the breast showing differences in volume focally using proprietary software (Vectra 3D, MirrorTM, Canfield) to map one breast onto the other to highlight these differences in volume—highlighting differences both regionally and globally post-operatively. This would then allow for calculation of volume difference (global) in cm3 between the treated and untreated breast and differences specifically in the quadrants (focal difference in volume) replaced by CWPFs between the two sides.
Conclusions
As interest in and patient uptake of CWPF for partial volume replacement increases, our knowledge gap of short and longer term outcomes mandates further research. Validated questionnaires provide clinicians with a useful insight into their patients’ satisfaction. The lack of a validated measurement tools in this patient population represents a gap in outcome assessment research. We are yet to identify the most suitable and accurate method of assessing these patients and for more robust numbers, such assessments need to be integrated within the patient care pathway to gauge both overall and aesthetic reported outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-23-21/rc
Data Sharing Statement: Available at https://asj.amegroups.com/article/view/10.21037/asj-23-21/dss
Peer Review File: Available at https://asj.amegroups.com/article/view/10.21037/asj-23-21/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-23-21/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the internal audit committee (Project Ref: BR2021_168) and further authorised via the University of East Anglia Ethical Committee. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Clough KB, Kaufman GJ, Nos C, et al. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol 2010;17:1375-91. [Crossref] [PubMed]
- Raja MA, Straker VF, Rainsbury RM. Extending the role of breast-conserving surgery by immediate volume replacement. Br J Surg 1997;84:101-5. [PubMed]
- Umar M, Jahangir N, Hughes M, et al. Incidence of shoulder functional morbidity following ipsilateral mastectomy and latissimus dorsi flap reconstruction. Acta Orthop Traumatol Turc 2019;53:448-51. [Crossref] [PubMed]
- Hamdi M. Oncoplastic and reconstructive surgery of the breast. Breast 2013;22:S100-5. [Crossref] [PubMed]
- Bertozzi N, Pesce M, Santi PL, et al. Oncoplastic breast surgery: comprehensive review. Eur Rev Med Pharmacol Sci 2017;21:2572-85. [PubMed]
- McCulley SJ, Schaverien MV, Tan VK, et al. Lateral thoracic artery perforator (LTAP) flap in partial breast reconstruction. J Plast Reconstr Aesthet Surg 2015;68:686-91. [Crossref] [PubMed]
- Quinn EM, O'Ceallaigh S, Highton L, et al. The Use of Local Perforator Flaps in Delayed or Secondary Breast Reconstruction. Plast Reconstr Surg Glob Open 2020;8:e3263. [Crossref] [PubMed]
- Kim JB, Kim DK, Lee JW, et al. The usefulness of pedicled perforator flap in partial breast reconstruction after breast conserving surgery in Korean women. Arch Plast Surg 2018;45:29-36. [Crossref] [PubMed]
- Agrawal SK, Shakya SR, Nigam S, et al. Chest wall perforator flaps in partial breast reconstruction after breast conservation surgery: an additional oncoplastic surgical option. Ecancermedicalscience 2020;14:1073. [Crossref] [PubMed]
- Rutherford CL, Barker S, Romics L. A systematic review of oncoplastic volume replacement breast surgery: oncological safety and cosmetic outcome. Ann R Coll Surg Engl 2022;104:5-17. [Crossref] [PubMed]
- Carmichael AR, Goyal A, Sami AS, et al. An evaluation of patient reported outcomes by utilizing breast Q following oncoplastic breast conserving surgery and arc-LICAP flap partial breast reconstruction. J Plast Reconstr Aesthet Surg 2021;74:1101-60. [Crossref] [PubMed]
- Orabi A, Youssef MMG, Manie TM, et al. Lateral chest wall perforator flaps in partial breast reconstruction. J Egypt Natl Canc Inst 2022;34:2. [Crossref] [PubMed]
- Mangialardi ML, Baldelli I, Salgarello M, et al. Thoracodorsal Artery Perforator Flap in Partial Breast Reconstruction: A Systematic Review. Plast Reconstr Surg Glob Open 2020;8:e3104. [Crossref] [PubMed]
- Roy PG, Mustata L, Hu J, et al. Partial Breast Reconstruction with Lateral Chest Wall Perforator Flap to Facilitate Breast Conservation in Breast Cancer: First 100 Cases with Cancer Outcomes at 8 Years Follow-Up and the Lessons Learned. Cancer Manag Res 2021;13:9453-66. [Crossref] [PubMed]
- BREAST-Q | Breast Cancer - Q-Portfolio Measuring What Matters to Patients. Available online: https://qportfolio.org/breast-q/breast-cancer/
- Cano SJ, Klassen AF, Scott AM, et al. A closer look at the BREAST-Q Clin Plast Surg 2013;40:287-96. [Crossref] [PubMed]
- Cano SJ, Klassen A, Pusic AL. The science behind quality-of-life measurement: a primer for plastic surgeons. Plast Reconstr Surg 2009;123:98e-106e. [Crossref] [PubMed]
- Dahlbäck C, Ullmark JH, Rehn M, et al. Aesthetic result after breast-conserving therapy is associated with quality of life several years after treatment. Swedish women evaluated with BCCT.core and BREAST-Q™. Breast Cancer Res Treat 2017;164:679-87. [Crossref] [PubMed]
- O'Connell RL, DiMicco R, Khabra K, et al. Initial experience of the BREAST-Q breast-conserving therapy module. Breast Cancer Res Treat 2016;160:79-89. [Crossref] [PubMed]
- Al-Ghazal SK, Sully L, Fallowfield L, et al. The psychological impact of immediate rather than delayed breast reconstruction. Eur J Surg Oncol 2000;26:17-9. [Crossref] [PubMed]
- Harris JR, Levene MB, Svensson G, et al. Analysis of cosmetic results following primary radiation therapy for stages I and II carcinoma of the breast. Int J Radiat Oncol Biol Phys 1979;5:257-61. [Crossref] [PubMed]
- Kovacs L, Eder M, Hollweck R, et al. New aspects of breast volume measurement using 3-dimensional surface imaging. Ann Plast Surg 2006;57:602-10. [Crossref] [PubMed]
- Moyer HR, Carlson GW, Styblo TM, et al. Three-dimensional digital evaluation of breast symmetry after breast conservation therapy. J Am Coll Surg 2008;207:227-32. [Crossref] [PubMed]
- O'Connell RL, Di Micco R, Khabra K, et al. The potential role of three-dimensional surface imaging as a tool to evaluate aesthetic outcome after Breast Conserving Therapy (BCT). Breast Cancer Res Treat 2017;164:385-93. [Crossref] [PubMed]
- Godden AR, Wood SH, James SE, et al. A scoring system for 3D surface images of breast reconstruction developed using the Delphi consensus process. Eur J Surg Oncol 2020;46:1580-7. [Crossref] [PubMed]
- Waljee JF, Hu ES, Ubel PA, et al. Effect of esthetic outcome after breast-conserving surgery on psychosocial functioning and quality of life. J Clin Oncol 2008;26:3331-7. [Crossref] [PubMed]
- Godden AR, O'Connell RL, Barry PA, et al. 3-Dimensional objective aesthetic evaluation to replace panel assessment after breast-conserving treatment. Breast Cancer 2020;27:1126-36. [Crossref] [PubMed]
- Kim KD, Kim Z, Kuk JC, et al. Long-term results of oncoplastic breast surgery with latissimus dorsi flap reconstruction: a pilot study of the objective cosmetic results and patient reported outcome. Ann Surg Treat Res 2016;90:117-23. [Crossref] [PubMed]
- Gao P, Bai P, Kong X, et al. Patient-Reported Outcomes and Complications Following Breast Reconstruction: A Comparison Between Biological Matrix-Assisted Direct-to-Implant and Latissimus Dorsi Flap. Front Oncol 2022;12:766076. [Crossref] [PubMed]
Cite this article as: Muktar S, Micha A, Farnworth S, O’Connell RL, Barry PA. Patient reported outcomes and aesthetic assessment following chest wall perforator flap partial volume replacement for primary breast cancer. AME Surg J 2023;3:49.