
Intraoperative presentation of coronary subclavian steal syndrome during coronary artery bypass surgery: a case report
Highlight box
Key findings
• Coronary subclavian steal syndrome (CSSS) may present intra-operatively as the development of haemodynamic instability on attempting to wean from cardiopulmonary bypass (CPB).
What is known and what is new?
• CSSS complicates up to 6.8% of coronary artery bypass grafting (CABG) operations, and classically presents several months post-operatively with symptoms ranging from stable angina associated with upper limb exertion to acute coronary syndrome and even sudden death.
• We report an unusual intra-operative presentation of CSS, likely triggered by the change in coronary resistance on weaning from CPB.
What is the implication, and what should change now?
• We advocate for a high degree of suspicion of CSSS in patients with significant atherosclerotic risk factors who experience similar difficulty in weaning from CPB.
• We propose a pre-operative screening algorithm that may allow for intervention prior to CABG to minimise operative risk.
Introduction
Background
The left internal mammary artery (LIMA) is the preferred conduit for coronary artery bypass surgery (CABG). Originating from the left subclavian artery, the LIMA is uniquely threatened by proximal subclavian artery stenosis (SAS). Coronary-subclavian steal syndrome (CSSS) describes the condition whereby haemodynamically significant proximal SAS results in flow limitation or reversal within the LIMA graft.
CSSS is estimated to complicate between 0.2–6.8% of CABG operations (1), with underlying SAS reported to be present in up to 5.3% of patients undergoing CABG, and in 11.8% of those with a history of peripheral vascular disease (2). CSSS typically presents several months post-operatively with symptoms ranging from stable angina to acute coronary syndrome associated with upper limb exertion and even sudden cardiac death (3).
Rationale and knowledge gap
To the best of our knowledge, there are only a limited number of reports describing intra-operative CSSS (4-6). Jelenc et al. described a case of acute limb ischaemia and LIMA flow reversal due to suspected intra-operative propagation of subclavian stenosis (4). Whilst LIMA flow reversal was confirmed using intra-operative transit-time graft flow measurement, there were no signs of myocardial ischaemia and the LIMA graft was left in place. Carrascal et al. described a case of sudden onset haemodynamic instability on weaning from cardiopulmonary bypass (CPB) due to a high flow fistula created by a prior subclavian-subclavian bypass ’sucking’ flow away from the nearby LIMA (5). Minami et al. similarly described a case of LIMA flow reversal due to the ’sucking’ of flow by a distal arteriovenous fistula in a haemodialysis patient (6). In both cases the LIMA anastomosis was taken down due to the intra-operative concerns. Though all cases highlight the risk of an intra-operative steal like phenomena in patients with significant vascular risk factors or altered anatomy, none describe the development of intra-operative myocardial ischaemia due to a proximal significant SAS.
Despite CSSS’s incidence and potentially devastating intra-operative and post-operative complications, current American and European revascularization guidelines do not detail recommendations for screening for SAS. However, screening and preventative measures have been discussed by other authors. Marshall et al. suggest all patients with clinical signs of peripheral vascular disease (e.g., carotid bruits) or a blood pressure differential of >15 mmHg should undergo invasive subclavian angiography for confirmation (7). In contrast, Takach et al. suggested best practice would be for all patients to undergo subclavian angiography alongside coronary angiography as part of the routine work-up for CABG (8).
Objective
We report an unusual intra-operative presentation of CSSS. We additionally propose a pre-operative screening algorithm that may allow for intervention prior to CABG, which could lead to improved post-operative outcomes. We present this case in accordance with the CARE reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-23-30/rc).
Case presentation
A 48-year-old Caucasian female was referred for urgent surgical revascularisation following admission with unstable angina, manifesting as progressively intrusive exertional chest tightness radiating to the left arm, with diaphoresis. Her admission troponin I was <17 ng/L. She had an abnormal lipid profile with elevated total cholesterol—7.7 mmol/L with a low-density lipoprotein cholesterol of 5.5 mmol/L and triglycerides of 2.1 mmol/L. She had suffered a non-ST elevation myocardial infarction 1 year prior and had her left main stem stented with a drug eluting stent. Her past medical history included: obesity, a right hip replacement, tonsillectomy and depression. She was an ex-smoker with a 15-pack-year history and had a strong family history of ischaemic heart disease with both parents suffering ischaemic heart disease in their 40’s. On examination she did not have any murmurs or signs of peripheral arterial disease. Radial artery pulses were recorded as being palpable bilaterally. Preoperative assessment revealed a normal Allens test with reperfusion of the hand in <5 seconds.
Her angiogram confirmed significant in-stent restenosis of the left main stem, with an instant flow reserve (iFR) 0.72, and a severe ostial left circumflex lesion. Echocardiogram confirmed good left ventricular function with ejection fraction 62% and no valvular pathology. In view of the good left ventricular function no viability testing was performed. The patients’ EuroSCORE II was calculated to be 1.87%
The patient underwent on-pump CABG ×2 in August 2019 on an arrested heart [vein graft to obtuse marginal 1 and LIMA to left anterior descending (LAD)]. The LIMA was harvested pedicled using cautery. Myocardial protection was performed with cold-blood cardioplegia administered antegrade into the aortic root, with repeat dose at 20 minutes and further cardioplegia delivered down the vein graft. Prior to establishing bypass, the pedicled LIMA flow was assessed visually as being satisfactory for use and was subsequently anastomosed to the LAD artery in its middle third. The vessel was approximately 2.0 mm and there was no mural disease at the site of the anastomosis. We do not have means of assessing the flow following anastomosis, however, there was myocardial contraction observed upon release of LIMA flow suggesting no technical issue with the anastomosis. The proximal anastomosis was performed following cross-clamp release, and the heart regained a normal sinus rhythm and was beating normally, not raising any suspicion of any problem. The heart was subsequently weaned from cardiopulmonary bypass (CPB), with no inotropic support. Shortly after, pronounced anterolateral ST-depression was observed followed by haemodynamic instability with hypotension not responsive to fluid or metaraminol boluses prompting going back onto bypass.
The LIMA-LAD anastomosis was taken down in view of the pattern of ischaemia. Good flow was observed from the LIMA and there was no evidence of a technical problem, however the decision was made to perform a vein graft to the LAD. The LIMA was not used as a free graft as there was concern that there may have been an injury during harvesting. The remainder of the procedure proceeded uneventfully. To further investigate, a CT-aortogram was performed which confirmed occlusion of the proximal left subclavian artery at its origin (Figure 1), suggesting the intraoperative picture was that of CSSS. She was referred to vascular surgery in view of the occluded left subclavian artery but no treatment was recommended by them following review of the images. She made an uneventful recovery and was discharged on the 7th postoperative day. She remains well at 4 years following surgery with no symptoms of angina. She has not had any imaging of her coronaries since surgery.
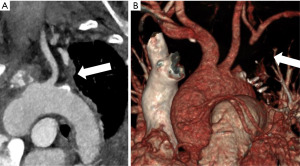
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee (Royal Papworth Hospital Research and Development) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Key findings
Here we report an unusual intra-operative presentation of CSSS, where a patient developed haemodynamic instability and pronounced ST-depression immediately following weaning from CPB during CABG, presumably representing myocardial ischaemia due to inadequate LIMA flow.
Strengths and limitations
Though it is difficult to be certain regarding the aetiology of the haemodynamic instability observed in our case, intra-operative assessment of the pedicled LIMA suggested satisfactory flow both prior to grafting and after the eventual take-down, with no evidence of any technical issue with graft harvesting or anastomosis. Considering these findings, the pattern of ischaemia in combination with the later discovery of significant SAS was strongly suggestive of CSSS. The ability to measure flow in the graft is not available at our centre but would have allowed us to confirm flow limitation or reversal in the LIMA graft.
Comparison with similar research
There are a limited number of case reports describing intra-operative CSSS (4-6), though none describe the development intra-operative myocardial ischaemia due to proximal significant SAS. Jelenc et al. described a case of intra-operative acute limb ischaemia; their patient presented with a pale limb with absent pulses and associated LIMA flow reversal, however, there was no haemodynamic instability, dynamic electrocardiogram (ECG) changes or troponin rise (4). Carrascal et al. and Minami et al. both describe cases presenting with intra-operative myocardial ischaemia necessitating subsequent LIMA take-down, however, neither case resulted from significant proximal SAS, with the proposed mechanism instead a high flow fistula diverting flow from the LIMA (5,6).
Explanations of findings
Like Carrascal et al. (5), we hypothesize that the presentation of CSSS in our case was triggered by the change in coronary vascular resistance upon weaning from CPB. With an unloaded heart on CPB, there is a low resistance vascular bed offering minimal resistance to LIMA flow throughout the cardiac cycle, but upon weaning from CPB there is increased coronary vascular resistance (9) and only diastolic perfusion which was presumably compromised due to the collateral origin of the LIMA.
Implications and actions needed
In view of the relatively high incidence of SAS in the CABG population, it is surprising that there are no recommendations on screening for this problem in guidelines on coronary revascularisation, especially in the era of increasing use of bilateral internal mammary arteries. There are two relatively inexpensive options for screening: (I) measurement of bilateral brachial blood pressure—where a difference in systolic pressure of 15 mmHg has been suggested to be diagnostic of haemodynamically significant SAS (10) with a sensitivity of 50% and specificity of 90% (the low sensitivity partly explained by the presence of bilateral SAS) (2); and (II) ultrasound doppler—where systolic vertebral artery flow reversal is suggestive of haemodynamically significant SAS. In patients with suspicion of SAS, diagnostic options include computed tomography (CT), magnetic resonance angiography or invasive angiography.
Following confirmation of SAS, the risk of subsequent CSSS has previously been mitigated via the use of venous grafts or a free LIMA graft (3). To enable use of the preferred pedicled LIMA options include brachiocephalic reconstruction alongside CABG or pre-operative endovascular stenting of SAS (8). Though surgical reconstruction was initially preferred, there is now growing evidence supporting pre-operative endovascular intervention, with Che et al. demonstrating excellent success rates (97.6%) with relatively low complication rates (death 0.6%, stroke 1.8%) and low rates of in stent re-stenosis (14.1%) in a study of 167 consecutive patients (11). Notably endovascular stenting is already the preferred treatment for established CSSS as per the most recent European Society of Cardiology (ESC) guidelines (12,13).
In our patient, subsequent measurement of bilateral brachial artery pressures identified a 30-mmHg difference in systolic pressure and would have alerted to the presence of SAS. As such we believe there may be some value in screening patients pre-operatively and are now introducing bilateral brachial blood pressure measurement as a routine screening for SAS in patients undergoing CABG and propose an algorithm (Figure 2).
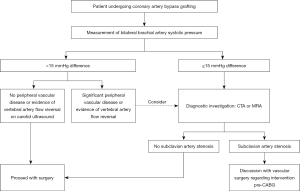
Conclusions
This case demonstrates an unusual intra-operative presentation of CSSS. We would recommend considering SAS particularly in patients with significant atherosclerotic risk factors and peripheral vascular disease. We propose a preoperative screening algorithm that may allow for identification of SAS and therefore potential intervention prior to patients undergoing CABG. This could lead to improved postoperative outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-23-30/rc
Peer Review File: Available at https://asj.amegroups.com/article/view/10.21037/asj-23-30/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-23-30/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee (Royal Papworth Hospital Research and Development) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Iglesias JF, Degrauwe S, Monney P, et al. Coronary Subclavian Steal Syndrome and Acute Anterior Myocardial Infarction: A New Treatment Dilemma in the Era of Primary Percutaneous Coronary Intervention. Circulation 2015;132:70-1. [Crossref] [PubMed]
- English JA, Carell ES, Guidera SA, et al. Angiographic prevalence and clinical predictors of left subclavian stenosis in patients undergoing diagnostic cardiac catheterization. Catheter Cardiovasc Interv 2001;54:8-11. [Crossref] [PubMed]
- De Roeck F, Tijskens M, Segers VFM. Coronary-subclavian steal syndrome, an easily overlooked entity in interventional cardiology. Catheter Cardiovasc Interv 2020;96:614-9. [Crossref] [PubMed]
- Jelenc M, Knezevic I, Stankovic M, et al. Intraoperative left subclavian artery occlusion with left hand ischaemia and steal syndrome in the left internal thoracic artery. Interact Cardiovasc Thorac Surg 2012;15:772-3. [Crossref] [PubMed]
- Carrascal Y, Arroyo J, Fuertes JJ, et al. Massive coronary subclavian steal syndrome. Ann Thorac Surg 2010;90:1004-6. [Crossref] [PubMed]
- Minami T, Uranaka Y, Tanaka M, et al. Coronary subclavian steal syndrome detected during coronary bypass surgery in a hemodialysis patient. J Card Surg 2015;30:154-6. [Crossref] [PubMed]
- Marshall WG Jr, Miller EC, Kouchoukos NT. The coronary-subclavian steal syndrome: report of a case and recommendations for prevention and management. Ann Thorac Surg 1988;46:93-6. [Crossref] [PubMed]
- Takach TJ, Reul GJ, Cooley DA, et al. Myocardial thievery: the coronary-subclavian steal syndrome. Ann Thorac Surg 2006;81:386-92. [Crossref] [PubMed]
- Belboul A, Rådberg G, Roberts D, et al. Intraoperative assessement of coronary flow and coronary vascular resistance during coronary bypass surgery. Scand Cardiovasc J 1999;33:23-8. [Crossref] [PubMed]
- Cua B, Mamdani N, Halpin D, et al. Review of coronary subclavian steal syndrome. J Cardiol 2017;70:432-7. [Crossref] [PubMed]
- Che WQ, Dong H, Jiang XJ, et al. Stenting for left subclavian artery stenosis in patients scheduled for left internal mammary artery-coronary artery bypass grafting. Catheter Cardiovasc Interv 2016;87:579-88. [Crossref] [PubMed]
- Lak HM, Shah R, Verma BR, et al. Coronary Subclavian Steal Syndrome: A Contemporary Review. Cardiology 2020;145:601-7. [Crossref] [PubMed]
- European Stroke Organisation. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2851-906. [Crossref] [PubMed]
Cite this article as: Mann S, Tweed K, Berman M, Ali JM. Intraoperative presentation of coronary subclavian steal syndrome during coronary artery bypass surgery: a case report. AME Surg J 2023;3:52.


