
Spontaneous resolution of Chiari malformation after bariatric surgery: a case report and literature review
Highlight box
Key findings
• Chiari malformation may spontaneously resolve with weight loss assisted by bariatric surgery avoiding the need for surgery.
What is known and what is new?
• Chiari malformation has been known to be associated with obesity.
• Our novel case report demonstrates the inverse relationship also holds true in which weight loss leads to improvement in Chiari malformation and cervical syrinx.
What is the implication, and what should change now?
• Weight loss should be considered as an adjuvant and reasonable treatment option for Chiari malformation.
• In asymptomatic obese patients with Chiari malformation, a period of conservative management with aggressive weight loss strategies may be considered.
Introduction
Chiari malformations represent a spectrum of congenital or acquired tonsillar abnormalities which may be associated with lumbar or cervical myelomeningoceles (1). The estimated incidence is 0.6–1% in the general population (2). Chiari I malformation is defined a greater than 5 mm of tonsillar descent beneath the foramen magnum and may be associated with a cervical syrinx (3). In the presence of tonsillar herniation, commonly reported symptoms include headaches which are exacerbated by Valsalva-type manoeuvres such as coughing, sneezing and laughing. When there is a symptomatic cervical syrinx, this usually manifests as syringomyelia with dissociated sensory loss and cervical myelopathy on examination.
Neurosurgical posterior fossa decompression is usually indicated in these symptomatic cases of syringomyelia manifesting as cape-like paraesthesia, motor deficit or even bulbar dysfunction (4). Given the absence or lack of recognition for other factors which may be favourable for medical management and resolution of syringes in the setting of Chiari I malformation, decompression has been recommended in symptomatic cases. We present a unique case of remarkable improvement of a cervical syrinx after weight loss from bariatric surgery and discuss the underlying pathophysiological principles. This is the first time there has been a clear temporal association reported between conservative management for a Chiari malformation with aggressive weight loss assisted by bariatric surgery which correlated with a clinical and radiological improvement in symptoms obviating the need for surgery. This novel demonstration posits that non-operative management and aggressive weight loss strategies in a carefully selected subgroup of overweight patients with a syrinx may obviate the need for surgery and its attendant risks. We present this case in accordance with the CARE reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-24-4/rc).
Case presentation
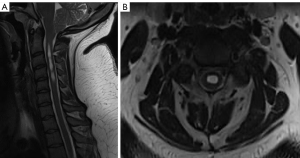
A 25-year-old female presented with suboccipital headaches worse on coughing and straining for several years prior to presentation which had been progressively worsening in the past 12 months. This was associated with reduced pain and temperature sensation in the left more than the right hand. Her past medical history was significant for a body mass index (BMI) of 55 kg/m2 awaiting bariatric surgery. On examination, she had signs of cervical myelopathy and dissociated sensory loss consistent with impaired spinothalamic function especially on the left side (Figure 1). There were no signs of bulbar dysfunction. An magnetic resonance imaging (MRI) brain and full spine demonstrated 7 mm of tonsillar descent beyond the foramen magnum and a significant cervical syrinx extending from C2–C7 (Figure 2).
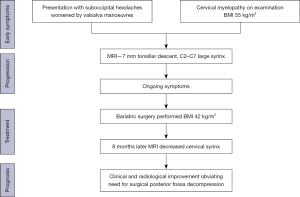

Given the significant peri-operative risks associated with her weight as well as increased surgical complexity, her bariatric surgery was expedited with a view to proceeding to a subsequent Chiari decompression following this. After successful bariatric surgery, however, with a reduction in BMI to 42 kg/m2, there was a significant improvement in all of her symptoms attributable to the cervical syrinx. This included improvement in her headaches as well as sensory loss. Furthermore, her gait disturbance improved as well. This was concordant with her MRI cervical spine 8 months later, which demonstrated markedly decreased syrinx in both length and volume with reduced extension to only between C2 and C4 (Figure 3). As such, it was deemed reasonable to proceed with ongoing conservative management.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Chiari malformation was first described by Hans Chiari in 1891 as descent of the cerebellar tonsils greater than 5 mm below the foramen magnum (5). This can be quantitatively defined as extent of tonsillar descent caudal to McRae’s line which joins the basin and opisthion (6). Anatomical predisposing factors for Chiari malformation include a shorter clivus or posterior fossa, smaller McRae line and less rounded atlantooccipital joints (7). The association of tonsillar descent with a spinal cord syrinx, especially in the cervical region, has the potential to cause significant neurological morbidity. This may include brainstem dysfunction, sensory disturbance, syringomyelia with disruption of the crossing spinothalamic tract fibres, as well as eventual spastic quadriparesis if long tracts are disrupted. As such, the current mainstay of treatment for symptomatic patients who have an extensive cervical syrinx is surgical decompression (1). This usually entails foramen decompression, C1 laminectomy and resection of the suboccipital ligament (8). Some authors would elect to perform dural opening, arachnoid dissection and adhesiolysis and tonsillar coagulation (8).
The exact pathophysiology of syrinx formation has prompted a number of historical theories (1,9). Williams et al. argued that Valsalva manoeuvres resulted in a unidirectional ‘distending’ downwards force driving cerebrospinal fluid (CSF) from the fourth ventricle through the obex and central canal into the syrinx (10,11). In other words, this was proposed to be a ‘communicating syringomyelia’. Gardner also subscribed to this proposal (12). However, high quality MRI and cadaveric dissections contradicted this proposal given the absence of a visible patent communication to support this theory (13). Subsequently, Oldfield et al. and Heiss et al. proposed a more complex explanation in which mechanical obstruction of CSF flow from the dorsal and lateral cerebromedullary cisterns through the foramen magnum occurs due to the tonsillar descent (14,15). During the cardiac systole, there is therefore dissociation between the cranial and spinal subarachnoid spaces with larger spinal subarachnoid pressure waves (14,15). It is this wave which drives CSF out of the subarachnoid space into the extracellular space of the spinal cord causing a syrinx to form over time (14,15). In other words, there is at least a partial obstruction of the usual subarachnoid space drainage pathways and an increase in the absolute and pulse pressure which creates a differential gradient (13,15). For this reason, Oldfield et al. coined this the ‘tonsillar piston theory’ which the pressure wave can progress even further caudally dissecting the spinal parenchyma to propagate the syrinx (15). This conversely also explains the predominant involvement of the cervical spinal cord given this bears the maximal pulsatile pressure (15).
This theory is consistent with the fundamental tenet that Chiari malformation associated with syrinx formation should be surgically decompressed to prevent further enlargement and subsequent neurological deficit (9,16). However, Killeen et al. astutely documented the natural history of patients with Chiari malformation and associated syrinx and found only 12% of patients developed progressive neurological deficit over a mean follow-up of 4.9 years (17). Of this small proportion of patients who became symptomatic, the most common manifestation was mild dysphagia only (69%) (17). Furthermore, the majority (63.8%) of patients who underwent surveillance only remained unchanged (17). Chavez et al. reinforced this conclusion by determining that 47.1% of patients improve without any surgical treatment (18). Additionally, only 26.5% noted worsening go their neurological symptoms with paraesthesia being the most likely complaint (45.6%) (18). The risk of developing a self-reported motor deficit was only 2.9% (18).
This was countered by Strahle et al. who examined a cohort of 147 patients suffering from Chiari I malformation (19). Over a mean duration of 4.6 years clinical follow-up, eight patients developed worsening of their syrinx (19). Despite this, the authors still concluded that the natural history of Chiari I malformation was relatively benign (19). What is clear is that whether the fundamental belief is to decompress all patients with a syrinx or monitor in the absence of clinical symptoms, it is vital to identify any potentially medical factors which may lead to the extremely rare case of spontaneous resolution of Chiari malformation and its associated syrinx (20-23). This has not only been in patients with clear favourable factors, such as elderly patients whose cerebellar tonsil atrophy or young children whose posterior fossa enlargement rationally coincides with resolution of syringomyelia (24-26). Cuthbert et al. proposed that normal CSF flow dynamics could be restored if a tear of fissure in the spinal cord after a Valsalva type manoeuvre thereby allowing the syrinx to decompress (27). It is therefore of interest to identify modifiable factors which may potentially obviate surgical decompression and its attendant risks (9,28).
Our novel case report of an obese patient with a Chiari malformation demonstrating remarkable self-reduction in size of a cervical syrinx was temporally associated with a significant amount of weight loss and improvement in BMI. From a pathophysiological perspective, obesity exacerbates the pressure gradient which precipitates syrinx formation given their raised intrathoracic and intraabdominal pressure (29,30). This is transmitted via the valveless vertebral venous compartments to the spinal subarachnoid space in turn and drives the increase in syrinx size (29). Previous reports of syrinx resolution have been reported in the setting of some form of disruption to the CSF drainage pathway (Tables 1,2). For example, Coppa et al. reported syrinx resolution after otorrhea requiring repair (21). Similarly, Miele et al. reported improvement after supratentorial craniotomy whilst Kilgore et al. noted this following pregnancy (22,44). These cases involved some indirect form of CSF pathway interference and in the case of parturition may be secondary to lysis of arachnoid scarring during labour (44). We present the first case of syrinx improvement following bariatric surgery.
Table 1
| Study | Country | Age, years | Sex | Initial BMI, kg/m2 |
Final BMI, kg/m2 |
Change in BMI, kg/m2 | Neurological symptoms |
|---|---|---|---|---|---|---|---|
| Arnautovic et al., 2013 (8) | Croatia | NA | Female | 28.5 | 41 | 12.5 | Yes |
| Arnautovic et al., 2013 (8) | Croatia | NA | Female | 19 | 28 | 9 | Yes |
| Arnautovic et al., 2013 (8) | Croatia | NA | Female | 45.3 | 36 | 9.3 | Yes |
| Avellino et al., 1996 (31) | USA | 5 | Male | NA | NA | NA | Yes (complex partial seizure) |
| Avellino et al., 1996 (31) | USA | 5 | Female | NA | NA | NA | Yes (hypesthesia pain, temperature), loss of reflexes, LL weakness and thoracic scoliosis |
| Briganti et al., 2013 (2) | Italy | 62 | Female | NA | NA | NA | Yes |
| Coppa et al., 2006 (21) | USA | 25 | Female | NA | NA | NA | Yes (pain, weakness and neck stiffness) |
| Cuthbert et al., 2021 (32) | UK | 25 | Female | NA | NA | NA | Yes (hypesthesia) |
| Deniz et al., 2009 (33) | Turkey | 41 | Male | NA | NA | NA | Yes (head and neck pain) |
| Di Rocco and Oi, 2005 (34) | Japan | 16 | Male | NA | NA | NA | Yes (but also headaches; nystagmus, bilateral hearing loss, peripheral facial palsy, dysphonia, bulbar palsy) |
| Fukutake and Hattori, 1998 (35) | Japan | 40 | Female | NA | NA | NA | Yes (thoracic girdle pain) |
| Gallo et al., 2021 (36) | USA | 46 | Female | NA | NA | NA | No |
| Gauge et al., 2012 (37) | UK | 49 | Female | NA | NA | NA | Yes (headaches) |
| Gaunt et al., 2016 (38) | UK | 58 | Female | NA | NA | NA | Yes (paraesthesia) |
| Guillen et al., 2004 (39) | Spain | 6 | Female | NA | NA | NA | No |
| Gupta et al., 2008 (40) | Canada | 9 | Male | NA | NA | NA | No |
| Jack et al., 1991 (41) | USA | 30 | Female | NA | NA | NA | Yes (areflexia; hypesthesia) |
| Jain et al., 2017 (42) | India | 32 | Female | NA | NA | NA | Yes (neck pain, paraesthesia) |
| Khanna and Coumans, 2016 (43) | USA | 57 | Female | NA | NA | NA | Yes (headache, UL paraesthesia) |
| Kilgore et al., 2022 (44) | USA | 30 | Female | NA | NA | NA | Yes (paraesthesia) |
| Klekamp et al., 2002 (20) | Germany | 37 | Female | NA | NA | NA | Yes (hypesthesia for light touch, pain and temperature) |
| Kyoshima et al., 2003 (45) | Russia | 39 | Male | NA | NA | NA | Yes (LL weakness, hypesthesia pain, light touch) |
| Mazumder et al., 2016 (46) | India | 5 | Female | NA | NA | NA | Yes (seizure) |
| Miele et al., 2012 (22) | USA | 31 | Female | NA | NA | NA | Yes (paraesthesia, diplopia, nystagmus) |
| Morioka et al., 1995 (47) | Japan | 25 | Female | NA | NA | NA | Yes (progressive neck pain, bilateral SDH) |
| Muthukumar and Christopher, 2013 (48) | India | 24 | Female | NA | NA | NA | Yes (headache, hoarse voice) |
| Olivero et al., 1992 (49) | USA | 28 | Female | NA | NA | NA | Yes (bilateral arm weakness) |
| Ramnarayan et al., 2018 (50) | India | 8 | Male | NA | NA | NA | No |
| Ramnarayan et al., 2018 (50) | India | 9 | Female | NA | NA | NA | Yes (headache, neck pain) |
| Santoro et al., 1993 (51) | Italy | 42 | Male | NA | NA | NA | Yes (hypesthesia, paraesthesia, gait disorder, hyperreflexia |
| Santoro et al., 1993 (51) | Italy | 31 | Female | NA | NA | NA | No |
| Sudo et al., 1990 (52) | Japan | 13 | Male | NA | NA | NA | Yes (areflexia, hyperreflexia, sensory deficit) |
| Sudo et al., 1990 (52) | Japan | 34 | Female | NA | NA | NA | NA |
| Sudo et al., 1990 (52) | Japan | 11 | Female | NA | NA | NA | NA |
| Sudo et al., 1990 (52) | Japan | 36 | Female | NA | NA | NA | NA |
| Sun et al., 2001 (53) | Canada | 11 | Male | NA | NA | NA | Yes (intermittent numbness of hand, seizures) |
| Sun et al., 2001 (53) | Canada | 7 | Male | NA | NA | NA | Yes (headache) |
| Sung et al., 2022 (54) | Australia | 43 | Female | NA | NA | NA | Yes (bilateral UL numbness) |
| Tokunaga et al., 2001 (55) | Japan | 5 | Female | NA | NA | NA | No |
| Tokunaga et al., 2001 (55) | Japan | 6 | Male | NA | NA | NA | No (sensory deficit) |
| Tokunaga et al., 2001 (55) | Japan | 7 | Male | NA | NA | NA | No (sensory deficit) |
| Tokunaga et al., 2001 (55) | Japan | 7 | Male | NA | NA | NA | No (sensory deficit) |
| Tokunaga et al., 2001 (55) | Japan | 8 | Male | NA | NA | NA | No (sensory deficit) |
| Tokunaga et al., 2001 (55) | Japan | 10 | Male | NA | NA | NA | No (sensory deficit) |
| Tokunaga et al., 2001 (55) | Japan | 10 | Male | NA | NA | NA | No (sensory deficit) |
| Tokunaga et al., 2001 (55) | Japan | 10 | Female | NA | NA | NA | No (sensory deficit) |
| Tokunaga et al., 2001 (55) | Japan | 10 | Male | NA | NA | NA | No (sensory deficit) |
| Tokunaga et al., 2001 (55) | Japan | 11 | Male | NA | NA | NA | No (sensory deficit) |
| Tokunaga et al., 2001 (55) | Japan | 13 | Female | NA | NA | NA | No (sensory deficit) |
| Tokunaga et al., 2001 (55) | Japan | 14 | Female | NA | NA | NA | Yes (headache) |
| Tokunaga et al., 2001 (55) | Japan | 15 | Female | NA | NA | NA | Yes (headache) |
| Tokunaga et al., 2001 (55) | Japan | 16 | Female | NA | NA | NA | No (sensory deficit) |
| Tortora et al., 2012 (56) | Italy | 24 | Male | NA | NA | NA | Yes (headaches) |
| Vaquero et al., 1990 (57) | Spain | 58 | Male | NA | NA | NA | Yes (paraesthesia, pain) |
| Vaquero et al., 1990 (57) | Spain | 36 | Female | NA | NA | NA | Yes (cervicobrachialgia) |
| Yuan et al., 2019 (58) | China | 36 | Female | NA | NA | NA | Yes (insensitivity to pain, hypalgesia, thermodypesthesia) |
| Our current case | Australia | 25 | Female | 55 | 42 | 13 | Yes |
| Median | NA | 24 | NA | 36.9 | 38.5 | 10.9 | NA |
NA, not available; LL, lower limbs; UL, upper limbs; SDH, subdural haematoma.
Table 2
| Study | Degree of tonsillar herniation | Associated syringomyelia | Neurological symptoms | Tonsillar herniation at follow-up | Syrinx at follow-up | Neurological symptoms at follow-up | Interventions | Interval time (months) |
|---|---|---|---|---|---|---|---|---|
| Arnautovic et al., 2013 (8) | Up to C1 | Yes | Yes | Yes | Yes | Worsened | NA | 48 |
| Arnautovic et al., 2013 (8) | Up to C1 | No | Yes | NA | NA | Worsened | Pregnancies | 36 |
| Arnautovic et al., 2013 (8) | Up to C1 | Yes | NA | NA | Resolution | Improved | Bariatric surgery | 6 |
| Avellino et al., 1996 (31) | Up to C1 | Yes | Yes | Yes | Reduced | Asymptomatic | None | 66 |
| Avellino et al., 1996 (31) | NA | Yes | Yes | No | Resolution | Improved | None | 96 |
| Briganti et al., 2013 (2) | Up to C1 | No | Yes | No | Resolution | Asymptomatic | NA | 48 |
| Coppa et al., 2006 (21) | Up to C1 | Yes | Yes | No | Resolution | Improved | Craniotomy + tympanomastoidectomy, VP shunt insertion |
NA |
| Cuthbert et al., 2021 (32) | Up to C1 | Yes | Yes | No | Resolution | Asymptomatic | NA | 28 |
| Deniz et al., 2009 (33) | Up to C1 | Yes | Yes | Yes | Reduced | Asymptomatic | NA | 132 |
| Di Rocco and Oi, 2005 (34) | Up to C2 | Yes | Yes | Yes | Reduced | NA | NA | 18 |
| Fukutake and Hattori, 1998 (35) | NA | Yes | Yes | NA | Resolution | Asymptomatic | None | 2 |
| Gallo et al., 2021 (36) | Up to C1 | Yes | Yes | No | Reduced | NA | MVC trauma | 192 |
| Gauge et al., 2012 (37) | Up to C1 | Yes | Yes | Yes | Resolution | Improved | Pegylated interferon and Ribavirin (Hep C) |
18 |
| Gaunt et al., 2016 (38) | Up to C1 | No | Yes | No | NA | Asymptomatic | NA | 67 |
| Guillen et al., 2004 (39) | Up to C1 | Yes | No | No | Resolution | Asymptomatic/unchanged | None | 96 |
| Gupta et al., 2008 (40) | Up to C1 | Yes | No | Yes | Reduced | Unchanged | Growth hormone replacement | 30 |
| Jack et al., 1991 (41) | Up to C1 | Yes | Yes | Yes | Resolution | Improved | None | 18 |
| Jain et al., 2017 (42) | Up to C1 | Yes | Yes | Yes | Reduced | Improved | NA | 30 |
| Khanna and Coumans, 2016 (43) | Up to C1 | Yes | Yes | Yes | Reduced | Reduced | NA (resolution of CF exacerbation) | 24 |
| Kilgore et al., 2022 (44) | Up to C1 | Yes | Yes | Yes | Reduced | Improved | Pregnancy, Medication | 13 |
| Klekamp et al., 2002 (20) | Up to C1 | Yes | Yes | No | Resolution | Asymptomatic | None | 32 |
| Kyoshima et al., 2003 (45) | Up to C1 | Yes | Yes | No | Reduced | Improved | None | 6 |
| Mazumder et al., 2016 (46) | Below C1 | Yes | Yes | No | Reduced | NA | NA | 12 |
| Miele et al., 2012 (22) | Up to C1 | Yes | Yes | No | Reduced | Asymptomatic | Supratentorial craniotomy for cavernoma | 72 |
| Morioka et al., 1995 (47) | Up to C1 | Yes | No | No | Resolution | Asymptomatic | NA | 2 |
| Muthukumar and Christopher, 2013 (48) | Up to C2 | Yes | Yes | No | Reduced | NA | NA (normal vaginal delivery) | 36 |
| Olivero et al., 1992 (49) | Up to C1 | Yes | Yes | Yes | Reduced | Improved | NA | 2 |
| Ramnarayan et al., 2018 (50) | NA | Yes | No | Yes | Reduced | Asymptomatic/unchanged | NA | 84 |
| Ramnarayan et al., 2018 (50) | NA | Yes | Yes | Yes | Reduced | Asymptomatic | NA | 84 |
| Santoro et al., 1993 (51) | Up to C1 | Yes | Yes | Yes | Reduced | Unchanged | None | 37 |
| Santoro et al., 1993 (51) | NA | Yes | No | Yes | Reduced | NA | Pregnancy/birth | 60 |
| Sudo et al., 1990 (52) | Up to C1 | Yes | Yes | No | Resolution | Improved | None | 27 |
| Sudo et al., 1990 (52) | NA | NA | NA | NA | Reduced | Improved | NA | 26 |
| Sudo et al., 1990 (52) | NA | NA | NA | NA | Reduced | Improved | NA | 65 |
| Sudo et al., 1990 (52) | NA | NA | NA | NA | Reduced | Improved | NA | 93 |
| Sun et al., 2001 (53) | Up to C1 | Yes | Yes | Yes | Reduced | Unchanged | None | 34 |
| Sun et al., 2001 (53) | Up to C1 | Yes | Yes | NA | Resolution | Asymptomatic | Diet | 72 |
| Sung et al., 2022 (54) | Up to C1 | Yes | Yes | Yes | Reduced | Improved | NA | 18 |
| Tokunaga et al., 2001 (55) | Yes | Yes | No | Yes | Reduced | Unchanged | NA | 24 |
| Tokunaga et al., 2001 (55) | Yes | Yes | No | No | Resolution | Unchanged | NA | 84 |
| Tokunaga et al., 2001 (55) | Yes | Yes | No | No | Reduced | Unchanged | NA | 60 |
| Tokunaga et al., 2001 (55) | Yes | Yes | No | Yes | Reduced | Unchanged | NA | 72 |
| Tokunaga et al., 2001 (55) | Yes | Yes | No | Yes | Resolution | Improved | NA | 96 |
| Tokunaga et al., 2001 (55) | NA | Yes | No | No | Resolution | Improved | NA | 36 |
| Tokunaga et al., 2001 (55) | Yes | Yes | No | No | Resolution | Improved | NA | 108 |
| Tokunaga et al., 2001 (55) | Yes | Yes | No | Yes | Resolution | Improved | NA | 24 |
| Tokunaga et al., 2001 (55) | NA | Yes | No | No | Resolution | Improved | NA | 96 |
| Tokunaga et al., 2001 (55) | Yes | Yes | No | Yes | Resolution | Improved | NA | 48 |
| Tokunaga et al., 2001 (55) | Yes | Yes | No | Yes | Reduced | Improved | NA | 24 |
| Tokunaga et al., 2001 (55) | Yes | Yes | Yes | Yes | Resolution | Improved | NA | 96 |
| Tokunaga et al., 2001 (55) | Yes | Yes | Yes | Yes | Reduced | Improved | NA | 48 |
| Tokunaga et al., 2001 (55) | Yes | Yes | No | No | Resolution | Unchanged | NA | 24 |
| Tortora et al, 2012 (56) | Up to C2 | Yes | Yes | No | Resolution | NA | NA | 36 |
| Vaquero et al., 1990 (57) | Up to C1 | Yes | Yes | Yes | Reduced | Unchanged | NA | 12 |
| Vaquero et al., 1990 (57) | NA | Yes | Yes | Yes | Reduced | Asymptomatic | NA | 24 |
| Yuan et al., 2019 (58) | Up to C1 | Yes | Yes | Yes | Reduced | Progressed | NA | 188 |
| Our current case | Up to C1 | Yes | Yes | Yes | Reduced | Improved | Bariatric surgery | 7 |
| Median | NA | NA | NA | NA | NA | NA | NA | 36 |
NA, not available; VP, ventriculoperitoneal; MVC, motor vehicle crash; CF, cardiac failure.
The nature of our case is even more striking given it is contrary to current literature. Smith et al. previously inspected a large series of 1,130 patients undergoing an MRI for any reason (59). These authors determined there was no relationship between BMI and level of cerebellar tonsillar descent (59). Furthermore, Eisenberg et al. argued that BMI was not associated with ectopia length given there was no significant difference in ectopia length between normal, overweight of obese patients (P>0.05) (60). Both of these reviews may be susceptible to selection bias given patients undergoing routine MRI are not necessarily reflective of the overall general population. More importantly, Arnautovic et al. specifically examined 60 consecutive adults with Chiari I malformation of whom 26 demonstrated a syrinx with a mean BMI of 30.35±7.65 kg/m2 (8). Importantly, vertical syrinx extension was greater in those overweight compared to those with a normal weight (P=0.027) (8). Indeed, for every BMI point gained there was a 0.14% increase in syrinx width (60). Moreover, gaining weight resulted in a syrinx formation in 2 patients (average BMI gain 10.8 points) which is the inverse occurrence of our patient (8).
In light of this, asymptomatic overweight patients with Chiari I malformation and an associated syrinx may be offered operative or non-operative management. There is a small risk of neurological deterioration whilst a rigorous weight loss regimen is implemented, but this is balanced against potentially avoiding perioperative surgical risks. This is especially crucial given obesity carries an increased anaesthetic risk especially when prone as Santiago et al. have noted (61). As such, these surgeons pioneered the semi-sitting position for posterior fossa decompressions in overweight patients finding that there is a lower bleeding risk and no increased risk of venous air embolism if appropriate preoperative echocardiography and patient selection is appropriately applied (61). It is also ironic that obesity increases the rate of obstructive sleep apnoea as does tonsillar herniation itself with brainstem compression given weakness of the pharyngeal and tensile laryngeal muscles as Amin et al. noted (62).
We have presented a rare case that supports medical management of Chiari I malformation with a cervical syrinx can occur. Bariatric surgery was the only major intervention implemented during this time course and is rationally attribute as the predominant reason for the improvement in the syrinx. This literature review also uncovered the contrary to hold true with Arnautovic et al. discovering patients whose weight gain led to syrinx formation (8). Limitations of the conclusion are secondary to the still primitive and ambivalent state of contemporary understanding of the exact pathophysiology of syrinx formation despite increasingly more logical theories. Spontaneous resolution is more common but this is the first time we have documented a potentially modifiable risk factor that could mitigate surgical risk for patients. Further multi-centre prospective randomized trials are required to progress our understanding.
Conclusions
In asymptomatic obese patients with a Chiari I malformation associated with a cervical syrinx, it may be reasonable to trial a period of conservative management with aggressive weight loss strategies including bariatric surgery. Conversely, overweight patients who have a known Chiari I malformation should be counselled on the importance of further weight gain and potential syrinx formation. Further prospective large multi-centre randomized trials are still required to validate our observations.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-24-4/rc
Peer Review File: Available at https://asj.amegroups.com/article/view/10.21037/asj-24-4/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-24-4/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosenblum JS, Pomeraniec IJ, Heiss JD. Chiari Malformation (Update on Diagnosis and Treatment). Neurol Clin 2022;40:297-307. [Crossref] [PubMed]
- Briganti F, Leone G, Briganti G, et al. Spontaneous resolution of Chiari type 1 malformation. A case report and literature review. Neuroradiol J 2013;26:304-9. [Crossref] [PubMed]
- Speer MC, Enterline DS, Mehltretter L, et al. Review Article: Chiari Type I Malformation with or Without Syringomyelia: Prevalence and Genetics. J Genet Couns 2003;12:297-311. [Crossref] [PubMed]
- Zisakis A, Sun R, Pepper J, et al. Chiari Malformation Type 1 in Adults. Adv Tech Stand Neurosurg 2023;46:149-73. [Crossref] [PubMed]
- Azahraa Haddad F, Qaisi I, Joudeh N, et al. The newer classifications of the chiari malformations with clarifications: An anatomical review. Clin Anat 2018;31:314-22. [Crossref] [PubMed]
- Barros DPM, Ribeiro ECO, Nascimento JJC, et al. Reliability and Agreement in the Cerebellar Tonsil Tip Localization: Two Methods Using the McRae Line Concept in MRI. World Neurosurg 2022;165:e611-8. [Crossref] [PubMed]
- Milhorat TH, Nishikawa M, Kula RW, et al. Mechanisms of cerebellar tonsil herniation in patients with Chiari malformations as guide to clinical management. Acta Neurochir (Wien) 2010;152:1117-27. [Crossref] [PubMed]
- Arnautovic KI, Muzevic D, Splavski B, et al. Association of increased body mass index with Chiari malformation Type I and syrinx formation in adults. J Neurosurg 2013;119:1058-67. [Crossref] [PubMed]
- Rusbridge C. New considerations about Chiari‐like malformation, syringomyelia and their management. In Practice 2020;42:252-67. [Crossref]
- Williams B. The distending force in the production of "communicating syringomyelia". Lancet 1969;2:189-93. [Crossref] [PubMed]
- Williams B. Further Thoughts on the Valvular Action of the Arnold‐Chiari Malformation. Developmental Medicine & Child Neurology 1971;13:105-12. [Crossref]
- Gardner WJ, Abdullah A, McCormack LJ. The varying expressions of embryonal atresia of the fourth ventricle in adults: Arnold-Chiari malformation, Dandy-Walker syndrome, arachnoid cyst of the cerebellum, and syringomyelia. J Neurosurg 1957;14:591-605. [Crossref] [PubMed]
- Oldfield EH, Muraszko K, Shawker TH, et al. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J Neurosurg 1994;80:3-15. [Crossref] [PubMed]
- Heiss JD, Patronas N, DeVroom HL, et al. Elucidating the pathophysiology of syringomyelia. J Neurosurg 1999;91:553-62. [Crossref] [PubMed]
- Oldfield EH. Pathogenesis of Chiari I - Pathophysiology of Syringomyelia: Implications for Therapy: A Summary of 3 Decades of Clinical Research. Neurosurgery 2017;64:66-77. [Crossref] [PubMed]
- Langridge B, Phillips E, Choi D. Chiari Malformation Type 1: A Systematic Review of Natural History and Conservative Management. World Neurosurg 2017;104:213-9. [Crossref] [PubMed]
- Killeen A, Roguski M, Chavez A, et al. Non-operative outcomes in Chiari I malformation patients. J Clin Neurosci 2015;22:133-8. [Crossref] [PubMed]
- Chavez A, Roguski M, Killeen A, et al. Comparison of operative and non-operative outcomes based on surgical selection criteria for patients with Chiari I malformations. J Clin Neurosci 2014;21:2201-6. [Crossref] [PubMed]
- Strahle J, Muraszko KM, Kapurch J, et al. Natural history of Chiari malformation Type I following decision for conservative treatment. J Neurosurg Pediatr 2011;8:214-21. [Crossref] [PubMed]
- Klekamp J. The pathophysiology of syringomyelia - historical overview and current concept. Acta Neurochir (Wien) 2002;144:649-64. [Crossref] [PubMed]
- Coppa ND, Kim HJ, McGrail KM. Spontaneous resolution of syringomyelia and Chiari malformation Type I in a patient with cerebrospinal fluid otorrhea. Case report. J Neurosurg 2006;105:769-71. [Crossref] [PubMed]
- Miele WR, Schirmer CM, Yao KC, et al. Spontaneous resolution of a Chiari malformation Type I and syrinx after supratentorial craniotomy for excision of a cavernous malformation. J Neurosurg 2012;116:1054-9. [Crossref] [PubMed]
- Williams A, Kamat A, Palmer J. Radiological demonstration of spontaneous resolution of type 1 Chiari malformation in a 17-year-old patient. Br J Neurosurg 2011;25:764-5. [Crossref] [PubMed]
- Castillo M, Wilson JD. Spontaneous resolution of a Chiari I malformation: MR demonstration. AJNR Am J Neuroradiol 1995;16:1158-60. [PubMed]
- Miller JH, Limbrick DD, Callen M, et al. Spontaneous resolution of Chiari malformation Type I in monozygotic twins. J Neurosurg Pediatr 2008;2:317-9. [Crossref] [PubMed]
- Morris CA, Forrester DA, Zanabrie R, et al. Spontaneous Unilateral Chiari I Secondary to Acquired Tonsillar Hypertrophy/Pseudomass With Syringomyelia in a Juvenile With Progressive Scoliosis. J Am Acad Orthop Surg Glob Res Rev 2023;7:e22.00111.
- Cuthbert H, Pepper J, Price R. Spontaneous resolution of a Chiari malformation with syringomyelia. BMJ Case Rep 2021;14:e241789. [Crossref] [PubMed]
- Teo DB. Images of the month 2: No laughing matter: Symptomatic Chiari malformation type I. Clin Med (Lond) 2022;22:368-9. [Crossref] [PubMed]
- Harold LR. What is the Relationship between Chiari I Malformation and Obesity. Arch Neurol & Neurosci 2018;1:101759143.
- Lam S, Auffinger B, Tormenti M, et al. The relationship between obesity and symptomatic Chiari I malformation in the pediatric population. J Pediatr Neurosci 2015;10:321-5. [Crossref] [PubMed]
- Avellino AM, Kim DK, Weinberger E, et al. Resolution of spinal syringes and Chiari I malformation in a child. Case illustration. J Neurosurg 1996;84:708. [Crossref] [PubMed]
- Cuthbert H, Pepper J, Price R. Spontaneous resolution of a Chiari malformation with syringomyelia. BMJ Case Rep 2021;14:e241789. [Crossref] [PubMed]
- Deniz O. Comorbid Panic Disorder and Chiari I Malformation: A Case Report.
- Di Rocco F, Oi S. Spontaneous regression of syringomyelia in Hajdu-Cheney syn-drome with severe platybasia. Case report. J Neurosurg 2005;103:194-7. [PubMed]
- Fukutake T, Hattori T. Reversible hydromyelia in a synchronised swimmer with re-current thoracic girdle pains. J Neurol Neurosurg Psychiatry 1998;65:606. [Crossref] [PubMed]
- Gallo P, Copley PC, McAllister S, et al. The impact of neurosurgical technique on the short- and long-term outcomes of adult patients with Chiari I malformation. Clin Neurol Neurosurg 2021;200:106380. [Crossref] [PubMed]
- Gauge N, Zebian B, Hoey A, et al. Chiari I malformation, syringomyelia and liver disease: an unusual resolution with implications for clinical practice. Clin Neurol Neurosurg 2012;114:686-8. [Crossref] [PubMed]
- Gaunt T, Aboelmagd S, Spohr H, et al. Spontaneous regression of a chiari malformation type 1 in a 58-year-old female. BJR Case Rep 2016;2:20160016. [Crossref] [PubMed]
- Guillen A, Costa JM. Spontaneous resolution of a Chiari I malformation associated syringomyelia in one child. Acta Neurochir (Wien) 2004;146:187-91. [Crossref] [PubMed]
- Gupta A, Vitali AM, Rothstein R, et al. Resolution of syringomyelia and Chiari malformation after growth hormone therapy. Childs Nerv Syst 2008;24:1345-8. [Crossref] [PubMed]
- Jack CR Jr, Kokmen E, Onofrio BM. Spontaneous decompression of syringomyelia: magnetic resonance imaging findings. Case report. J Neurosurg 1991;74:283-6. [Crossref] [PubMed]
- Jain PK, Sreeharsha SV, Dugani S. Spontaneous resolution of syringomyelia in Chiari I malformation: A review of literature. Neurol India 2017;65:1187-9. [Crossref] [PubMed]
- Khanna AR, Coumans JV. Spontaneous Improvement of Chiari I Malformation and Syringomyelia in a Patient With Cystic Fibrosis: Case Report. Neurosurgery 2016;78:E305-8. [Crossref] [PubMed]
- Kilgore MD, Mathkour M, Dunn RH, et al. Spontaneous resolution of syringomyelia following pregnancy and parturition in a patient with type I chiari malformation: A case and systematic review. Clin Neurol Neurosurg 2022;222:107413. [Crossref] [PubMed]
- Kyoshima K, Bogdanov EI. Spontaneous resolution of syringomyelia: report of two cases and review of the literature. Neurosurgery 2003;53:762-8; discussion 768-9. [Crossref] [PubMed]
- Mazumder AK, Das S, Krishnan P. Spontaneous resolution of Chiari malformation and associated syringomyelia. Neurol India 2016;64:1335-6. [Crossref] [PubMed]
- Morioka T, Shono T, Nishio S, et al. Acquired Chiari I malformation and syringomyelia associated with bilateral chronic subdural hematoma. Case report. J Neurosurg 1995;83:556-8. [Crossref] [PubMed]
- Muthukumar N, Christopher J. Spontaneous resolution of Chiari I malformation and associated syringomyelia following parturition. Acta Neurochir (Wien) 2013;155:817-8. [Crossref] [PubMed]
- Olivero WC, Dinh DH. Chiari I malformation with traumatic syringomyelia and spontaneous resolution: case report and literature review. Neurosurgery 1992;30:758-60. [Crossref] [PubMed]
- Ramnarayan R, Ganesh CVS, Kumar R. Spontaneous Resolution of Chiari 1-Associated Syringomyelia: A Report of Two Cases. Pediatr Neurosurg 2018;53:238-42. [Crossref] [PubMed]
- Santoro A, Delfini R, Innocenzi G, et al. Spontaneous drainage of syringomyelia. Report of two cases. J Neurosurg 1993;79:132-4. [Crossref] [PubMed]
- Sudo K, Doi S, Maruo Y, et al. Syringomyelia with spontaneous resolution. J Neurol Neurosurg Psychiatry 1990;53:437-8. [Crossref] [PubMed]
- Sun PP, Harrop J, Sutton LN, et al. Complete spontaneous resolution of childhood Chiari I malformation and associated syringomyelia. Pediatrics 2001;107:182-4. [Crossref] [PubMed]
- Sung PS, Oh JS, Choi JI. Acute Budd-Chiari syndrome with thrombotic thrombocytopenia after BNT162b2 mRNA vaccination. Liver Int 2022;42:1447-8. [Crossref] [PubMed]
- Tokunaga M, Minami S, Isobe K, et al. Natural history of scoliosis in children with syringomyelia. J Bone Joint Surg Br 2001;83:371-6. [Crossref] [PubMed]
- Tortora F, Napoli M, Caranci F, et al. Spontaneous regression of syringomyelia in a young patient with Chiari type I malformation. Neuroradiol J 2012;25:593-7. [Crossref] [PubMed]
- Vaquero J, Martínez R, Arias A. Syringomyelia-Chiari complex: magnetic resonance imaging and clinical evaluation of surgical treatment. J Neurosurg 1990;73:64-8. [Crossref] [PubMed]
- Yuan C, Yao Q, Zhang C, et al. Spontaneous Resolution of Syringomyelia with a 16-Year Serial Magnetic Resonance Imaging Follow-Up: A Case Report and Literature Review. World Neurosurg 2019;130:432-8. [Crossref] [PubMed]
- Smith BW, Strahle J, Kazarian E, et al. Impact of body mass index on cerebellar tonsil position in healthy subjects and patients with Chiari malformation. J Neurosurg 2015;123:226-31. [Crossref] [PubMed]
- Eisenberg L, Gienapp AJ, Eisenberg A, et al. Effect of Body Mass Index on Chiari Malformation 1 Tonsil Ectopia Length in Adults. World Neurosurg 2023;176:e380-3. [Crossref] [PubMed]
- Bastianon Santiago R, Kaye B, Hagerty V, et al. Chiari Malformation Type 1 and Semi-Sitting Position-A Suitable Alternative for Patients with High BMI. World Neurosurg 2023;177:e433-9. [Crossref] [PubMed]
- Amin R, Sayal P, Sayal A, et al. The association between sleep-disordered breathing and magnetic resonance imaging findings in a pediatric cohort with Chiari 1 malformation. Can Respir J 2015;22:31-6. [Crossref] [PubMed]
Cite this article as: Kweh BTS, Seo J, Mikhail R, Khoo B, Gupta A, Donaldson C, Asaid M, Gonzalvo AC. Spontaneous resolution of Chiari malformation after bariatric surgery: a case report and literature review. AME Surg J 2024;4:5.


