
Optimizing retrosigmoid approach for giant vestibular schwannoma resection: strategies and advances in surgical technique
Highlight box
Surgical highlights
• Vestibular schwannoma (VS) excision using the retrosigmoid approach in the lateral Fukushima position with the assistance of intraoperative neuromonitoring and fluorescein sodium (FS).
What is conventional and what is novel/modified?
• Predicting the origin of the VS preoperatively may help in the prediction of facial nerve position intraoperatively.
• The use of intraoperative FS helps in differentiation between tumor cells and normal tissue intraoperatively.
• The use of handheld 2µ-thulium flexible laser fiber helps in capsule opening and tumor debulking with good control and homeostasis, also helps in dura removal for opening of the internal auditory canal.
• The use of the Sonopet for internal auditory canal opening is useful and safe.
• The use of flexible endoscope for intraoperative checking of any remnant is useful for total resection.
What is the implication, and what should change now?
• The use of all new technologies and advancements available makes a difference in the outcome and we encourage all the surgeons to keep updated with every technique, instrument and tool that can make difference for the best of the patients.
Introduction
Vestibular schwannomas (VSs), also known as acoustic neuromas, are benign, well-capsulated, and slow-growing tumors that arise from Schwann cells, which form the myelin sheath of the superior or inferior vestibular nerve. According to the Koos’ classification system, Grade IV VSs are characterized by their large and giant size (longitudinal diameter exceeding 3 cm), which can compress the brainstem and displace the fourth ventricle rather than the adjacent cranial nerves.
According to the algorithm of treatment for VS (1), the treatment of giant VS is surgical resection (Figure 1); even in patients older than 70 years, a judicious debulking is still the choice. Treatment aims to establish tumor control while preserving the quality of life through the preservation of the facial nerve or preservation of cochlear nerve if possible in cases of serviceable hearing pre-operatively, as giant VS compress the adjacent structures of nerves, brainstem, cerebellum, and fourth ventricle if large enough (2,3).

Three surgical approaches have been established for the management of VSs: the retrosigmoid approach, translabyrinthine approach, and middle fossa approach. The retrosigmoid approach is the most commonly employed technique, and is typically indicated for both small and large tumors, as well as for cases where hearing preservation is a primary goal. The translabyrinthine approach, on the other hand, is reserved for patients who have already experienced significant hearing loss, as it is often used to treat larger tumors as it gives a direct access to the tumor with full visualization of facial nerve course, but it is not recommended when the patient has high jugular bulb as it limits drilling borders and decrease working space window. The middle fossa approach, although less frequently utilized, is a viable option for managing small intracanalicular tumors when preserving hearing is a key objective of the surgical procedure (1). We will discuss the retrosigmoid approach in steps with images and illustrated video for the steps listed below, as it gives direct access to the tumor with a good control of the neurovascular structure in the cerebellopontine angle (CPA), also it has shorter operation time and more familiar for surgeons.
Surgical removal of giant VS is a challenging procedure that requires a multidisciplinary team approach and careful preoperative planning. The surgical techniques and outcomes for patients with VS have improved using intraoperative neuromonitoring, flexible endoscopes, intraoperative fluorescein sodium (FS), and minimally invasive approaches (1,4). This study explores the technological advancement and surgical techniques from the author’s papers and experience with VS surgery. We present this article in accordance with the SUPER reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-24-3/rc).
Preoperative preparations and requirements
All procedures performed in this study were in accordance with the ethical standards of the local Ethics Committee (IRB) of the Hospital (Lazio1, ASLRoma1) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients before surgery for publication of this manuscript, the accompanying images, and the video. A copy of the written consent is available for review by the editorial office of this journal.
- Assessing general medical condition and fitness for anesthesia according to the American Society of Anesthesiology (ASA) clinical classification (2,3). It is contraindicated for patients who have ASA clinical classification >3 to perform the surgery due to the high risk of anesthesia.
- Basic laboratory panel: complete blood count (CBC), electrolytes, kidney function test (KFT), prothrombin time (PT), partial thromboplastin time (PTT), prepare two units of packed red blood cells.
- Radiology: brain magnetic resonance imaging (MRI) with contrast, brain computed tomography (CT).
- Examples of audiological testing include monosyllabic speech audiograms, pure tone audiometry (PTA), and auditory brainstem response (ABR)—used intraoperatively. The results are interpreted using the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) hearing classification field (2,3). PTA and speech discrimination score (SDS) evaluations form the basis of the AAO-HNS hearing classification. Evaluation of the function of the facial nerve (CN VII) using the House-Brackmann (HB) grading system (2,3).
Team members: a main surgeon and an assistant surgeon, anesthesiologist, neurophysiologist, scrubbing nurse and circulating nurse, and an intensivist for post operative care (Video S1).
Step-by-step description
Regarding giant VS, the retrosigmoid approach is commonly performed as it offers direct access to and good control of neurovascular structures in the CPA; we will summarize it in steps with the important notes for every step.
Position
The patient is placed in a lateral Fukushima position, as seen in Figure 2. The body is placed obliquely on the table with the back at the edge; the lower shoulder is placed at 90 degrees to the body with a silicon axilla pad, and the upper arm at 45 degrees with traction to the shoulder away from the head to give space for the main operator’s hands. The head is fixed in a position of minimal rotation to the opposite side, flexed, and the mastoid is the highest point and is parallel to the floor.

Table 1 compares the different positions used with the advantages and disadvantages.
Table 1
| Position | Advantages | Disadvantages |
|---|---|---|
| Semi-sitting | (I) Improves drainage of blood and CSF, reducing blood during craniotomy and dissection. (II) Allows for bimanual dissection technique without continuous suction. (III) Gravity retracts the cerebellar hemispheres, reducing need for retraction (5,6) | (I) Facilitates air aspiration into blood vessels, causing VAE. (II) Increases risk of tension pneumocephalus and PAE. (III) May not be suitable for all patients due to potential risks (5,6) |
| Supine | Lower risk of VAE compared to semi-sitting position (6) | May have higher blood loss compared to semi-sitting position (6) |
| Lateral Fukushima | Shorter operation time compared to semi-sitting position. Around 4 hours (7) | Need for continuous suctioning of blood and irrigation of fluid during the surgery |
CSF, cerebrospinal fluid; VAE, venous air embolism; PAE, paradoxical air embolism.
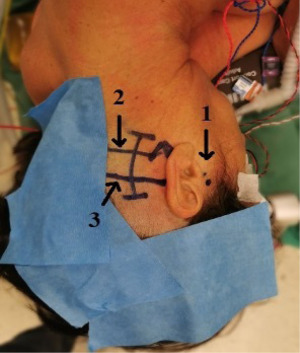
Skin incision
As seen in Figure 3, the skin landmarks are the root of the zygoma, superior nuchal line (SNL), inferior nuchal line (INL), mastoid tip, and digastric groove (DG). We draw a slightly curved line behind the mastoid, 1 cm above the SNL, and 1 cm below the INL. After the skin incision, a free pericranial flap (3 cm × 3 cm) is harvested for dura closure. During the remainder of the procedure, the graft patch is soaked and stored in a gentamycin-enriched saline solution (8).
FS
At the time of skin incision, FS is administered at a dose of 5 mg/kg intravenously. A specialized filtered microscope is then employed to visualize the enhancement of fluorescence in the tumor cells in yellowish-green color, which allows for differentiation from non-affected nerves, which appear white.
Keyhole retrosigmoid craniotomy
Subperiosteal dissection to expose the DG inferiorly and the asterion superiorly and 4–5 cm posterior to that. The craniotomy border anteriorly is the line connecting the asterion and the beginning of the DG. A 3 cm × 3 cm craniotomy is performed using a 4-mm extra-coarse diamond drill (9). After exposing the intact dura, a 5-mm longitudinal groove is made at the posterior border of the mastoid body, safely exposing the sigmoid sinus anteriorly as well as the junction of the sigmoid sinus and the transverse sinus, then inferiorly along the inferior edge (suboccipital groove). In case of opening mastoid air cells while drilling, it is preferred to seal it with bone wax before moving to the next step.
Lateral medullary cistern cerebrospinal fluid (CSF) aspiration and dural opening
Small dura opening inferiorly and toward the foramen magnum. We open the arachnoid membrane of the lateral medullary cistern, and then CSF is aspirated slowly for cerebellar relaxation (10). Then dura is opened in a semi-circular shape, covering part of the cerebellar hemisphere to protect it during retraction.
Internal auditory canal opening
As seen in Figure 4, the dura posterior to porus acusticus is removed either incised or by laser (10,11) (handheld 2µ-thulium flexible laser fiber, Revolix jr.®, Lisa laser USA, Pleasanton, CA, USA) in an inverted U shape fashion. The dura landmark (12) is from the Tübingen line to the supramental tubercle and extends about 6–8 mm toward the fovea. The Sonopet Ultrasonic Aspirator (Stryker Inc., Kalamazoo, MI, USA) or drill is used to expose the canal; in case of using the drill, the internal auditory canal is exposed using a 4-mm coarse diamond burr, and the lateral boundaries of the canal are defined with progressively smaller diamond burrs. The facial and cochlear nerves must be exposed by removing the bone from the upper and lower corners of the porus acoustics, as the tumor usually compresses these nerves as it protrudes from the internal auditory canal.

The usage of drill or Sonopet depends on the surgeon preference, availability, and experience.
Intraoperative neuromonitoring for facial nerve
The most important step for removal safety is to use the neuromonitoring frequently before, while, and after tumor excision. It consists both free-running electromyography (EMG) recording and direct electrical stimulation. When the facial nerve is stimulated, a monopolar or bipolar stimulator is used, with currents ranging from 2 mA or higher (on the capsule for localizing the nerve course) to 0.3–0.05 mA (directly on the nerve for function confirmation) (13,14), and if facial nerve was intact and functioning well you could receive stimulation even at 0.007 mA. The facial nerve is expected to be located anterior (31–52%), anterosuperior (38.5–48%), anteroinferior (5.3–21%), or, very rarely, dorsal (0.3–3.8%) to the tumor, based on the growth direction of the tumor and it might be variable (1).
A final-to-baseline facial motor evoked potential (FMEP) amplitude ratio is also observed and reduction of 50% at the end of the surgery has been identified as a good predictor for postoperative CN VII outcome (15).
Intraoperative monitoring of the cochlear nerve is usually performed by evoking and recording ABR in case of serviceable hearing pre operatively. Lower cranial nerves and trigeminal nerve are also monitored.
Capsular elevation and tumor debulking
Starting from the dorsal surface of the tumor capsule is elevated using the V-cut technique (Fukushima technique) using a laser fiber (10,11) or micro scissor. After that, we remove the tumor from inside piece by piece with intermittent neuromonitoring to leave an outer residual around 2–3 mm using sharp micro scissors and an ultrasonic aspirator (ideal setting: power 50, suction 5, irrigation 5). After reducing the internal tumor volume creates a more pliable structure that is easily manipulated and dissected from the facial nerve and adjacent structures.
Tumor removal from the internal auditory canal
Start with the removal of the visible part under the microscope field, and the instrument of choice is the McElveen-Hitzelberger neural dissector to remove the remnant part, cut it from the nerve of origin and dissection from the surrounding nerves piece by piece. Also, a 1-mm ring or cup curette, or Crabtree right angle dissector can be used.
Flexible endoscopic check of the internal auditory canal
Using the flexible video endoscope in microsurgery helps search for any remnant tumor in the fundus that is not visible by the straight microscope and checking if there is any open air-cell while opening the canal that has to be closed with bone wax. The endoscope is a 4-mm flexible video endoscope (4 mm × 65 cm, Karl Storz SE & Co. KG, Tuttlingen, Germany) (16).
Closure
After removal of the tumor and checking the facial nerve functionality, injection of diluted papaverine in normal saline solution in the cistern cavity for 1 minute to prevent vasospasm (17). Then, wash with saline and ensure homeostasis. The defect is filled with the harvested autologous pericranium graft for dura closure as an underlay hourglass-shaped plug (8). Next, cover it with a dural sealant (DuraSeal, Covidien LLC, Mansfield, MA, or Tisseel, Baxter, Deerfield, IL, USA) and small pieces of the surgical patch (TachoSil®, Takeda, Japan). Reinstall the autologous bone that was previously removed using mini plates and mini screws. HydroSetTM bone cement (Stryker Inc.) can be used to fill the residual defect (8). The wound is closed by layers.
Postoperative considerations and tasks
After the operation, all patients need an intensive care unit for close monitoring and basic laboratory panel: CBC, KFT, and electrolytes. Brain CT post-operative day 1 and an MRI with contrast at 3 months post-operation. The first follow-up is at 2 weeks then at 6 months.
The patient could be transferred to the floor in case his vital signs were within normal limits, fully conscious and oriented (Glasgow coma score is 15); his ordered labs are normal, no pain, no motor weakness related to the procedure, and his CT scan was reassuring that there is no bleeding or cerebellum edema.
Possible serious complications include: bleeding which needs a serious intervention and close observation and a possible reopening to control it, also cerebellum edema due to venous infarction is a very serious complication that has to be managed carefully due to the possibility of ventricular compression and hydrocephalus or brainstem compression and ischemia, infection is a serious complication that needs intervention and treatment according to the case situation.
Tips and pearls
In the positioning of the patient, traction to the shoulder away from the head to give space for the main operator’s hands. During skin incision, the harvested graft patch is soaked and stored in gentamycin-enriched saline solution till it is used in the closure. In case of opening mastoid air cells while drilling, it is preferred to seal them with bone wax before moving to the next step so you remember them later on. When draining CSF from the lateral medullary cistern, it is essential to avoid active suctioning, allowing the fluid to drain naturally and passively. Continuous facial nerve neuromonitoring with intermittent stimulation increases your safety margins during resection. After reducing the internal tumor volume creates a more pliable structure that is easily manipulated and dissected from the facial nerve and adjacent structures. The weakest point of the facial nerve is at the entry point of the meatus against the bone, and it is the most adherent part of the tumor; usually, it has to be dissected carefully.
Discussion
Surgical resection of giant VSs poses a significant challenge, particularly in older patients with comorbidities, where thoughtful consideration of various factors is necessary to achieve the primary goal of surgery: maximal safe resection. In this context, prudent decision-making is essential to balance the benefits of complete tumor removal with the risks associated with prolonged surgical time, blood loss, and postoperative complications in vulnerable patients.
Each stage of the retrosigmoid approach for VS resection is critical and requires meticulous attention to detail, as it is associated with its own set of considerations, potential complications, and outcomes.
Facial nerve function preservation
Kohno et al. (18) identified 3 probable planes for tumor dissections: (I) intracapsular; (II) sub-perineural (subcapsular); and (III) subarachnoid. The ideal cleavage plane for facial nerve and cochlear nerve functions preservation during VS microsurgery is the sub-perineural plane according to Sasaki et al. (19).
The relevance of experience in VS microsurgery was highlighted by a review of 108 consecutive cases by Nutik et al. (20), who found that anatomic preservation of facial nerve was inversely related to tumor size and improved as the series continued. According to Troude et al. (21), postoperative early facial nerve outcome is the strongest predictor of long-term facial nerve function. In the same multivariate analysis, a prior surgery had a negative impact on facial outcome. Mastronardi et al. (22) found that the following conditions were associated with a 71.7% better outcome when analyzing the facial nerve results in 60 consecutive cases: solid tumors, the presence of preoperative trigeminal symptoms, the presence of preoperative ataxia, the absence of tight tumor capsule adhesions, low-bleeding tumors, use of endoscopically assisted microsurgery, and total/near total tumor removal. While prior radiation has an adverse effect on facial nerve outcome.
Predicting facial nerve position
Preoperative audiometric and vestibular assessments, including vestibular evoked myogenic potentials (VEMPs), caloric stimulation test, and PTA, have been shown to predict the origin of the vestibular tumor with high accuracy, allowing for the potential identification of the possible position of the facial nerve (23). This information can be utilized to minimize the risk of facial nerve damage during surgery.
Using intraoperative FS
In a comparative analysis for the use of FS in VS surgery (4), it found that the use of FS-assisted surgery for VS resection has a significant effect on the extent of tumor removal, whereas it does not influence postoperative hearing outcomes or facial nerve function. Furthermore, FS has been demonstrated to be safe and well-tolerated in VS surgery.
Surgical dissection for larger, vascularized and adherent VS
Large, vascularized VS pose a challenging microsurgical challenge, especially when the capsule is attached to nearby neural structures, such as the brainstem and facial nerve. Numerous authors observed that partial surgical removal required close long-term follow-up to look for potential recurrences (24,25). The hypervascular and adherent characteristics of giant VS may be caused by a number of mechanisms, which may provide targets for future treatment.
Cystic VS
Cystic VS did not exhibit indicators of higher adhesion intraoperatively or significantly impact the postoperative result, according to various authors (26-28). However, Mastronardi et al. (22) observed tight adhesion between the capsule and nerve tissues in large cystic VS cases.
Complications
Results in 60 consecutive cases by Mastronardi et al. (22) showed that mortality was zero, permanent complication 3.3% (represented by abducens nerve paralysis and obstructive hydrocephalus), transient complications 15%, re-growth rate was 13.3% and all of them had sub-total or partial removal.
Conclusions
The best course of treatment for giant VS is surgical resection. Even in the case of older patients, it should be taken into consideration because it has a positive impact on the patients’ quality of life.
Giant VS is a surgical challenge in order to obtain the right balance between function preservation and maximum tumor removal. The retrosigmoid approach is safe and feasible to remove large and giant VS, as shown by the acceptable postoperative complication rates.
Surgical experience, anatomical knowledge, appropriate instruments with neuromonitoring, and team work are all an important key factor for better outcome.
The surgical highlights include the meticulous application of the retrosigmoid approach, precise patient positioning, careful dissection of the tumor capsule, continuous facial nerve neuromonitoring, and thorough postoperative monitoring to achieve optimal outcomes in giant VS surgery.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-24-3/rc
Peer Review File: Available at https://asj.amegroups.com/article/view/10.21037/asj-24-3/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-24-3/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the local Ethics Committee (IRB) of the Hospital (Lazio1, ASLRoma1) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients before surgery for publication of this manuscript, the accompanying images, and the video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mastronardi L, Fukushima T, Campione A. Advances in Vestibular Schwannoma Microneurosurgery: Improving Results with New Technologies. Springer; 2019.
- Turel MK, D'Souza WP, Chacko AG, et al. Giant vestibular schwannomas: Surgical nuances influencing outcome in 179 patients. Neurol India 2016;64:478-84. [Crossref] [PubMed]
- Turel MK, Thakar S, Rajshekhar V. Quality of life following surgery for large and giant vestibular schwannomas: a prospective study. J Neurosurg 2015;122:303-11. [Crossref] [PubMed]
- Alomari AA, Eid SS, Fraschetti F, et al. Comparative Analysis on Vestibular Schwannoma Surgery with and without Intraoperative Fluorescein Sodium Enhancement. Brain Sci 2024;14:571. [Crossref] [PubMed]
- Duke DA, Lynch JJ, Harner SG, et al. Venous air embolism in sitting and supine patients undergoing vestibular schwannoma resection. Neurosurgery 1998;42:1282-6; discussion 1286-7. [Crossref] [PubMed]
- Rath GP, Bithal PK, Chaturvedi A, et al. Complications related to positioning in posterior fossa craniectomy. J Clin Neurosci 2007;14:520-5. [Crossref] [PubMed]
- Spektor S, Fraifeld S, Margolin E, et al. Comparison of outcomes following complex posterior fossa surgery performed in the sitting versus lateral position. J Clin Neurosci 2015;22:705-12. [Crossref] [PubMed]
- Mastronardi L, Cacciotti G, Caputi F, et al. Underlay hourglass-shaped autologous pericranium duraplasty in "key-hole" retrosigmoid approach surgery: Technical report. Surg Neurol Int 2016;7:25. [Crossref] [PubMed]
- Sameshima T, Mastronardi L, Friedman AH, et al. Fukushima's microanatomy and dissection of The temporal Bone: for surgery of acoustic neuroma, and petroclival meningioma. 2007. Available online: https://cir.nii.ac.jp/crid/1130000794979828992
- Mastronardi L, Cacciotti G, Roperto R, et al. How I Do It: The Role of Flexible Hand-held 2µ-Thulium Laser Fiber in Microsurgical Removal of Acoustic Neuromas. J Neurol Surg B Skull Base 2017;78:301-7. [Crossref] [PubMed]
- Mastronardi L, Cacciotti G, Scipio ED, et al. Safety and usefulness of flexible hand-held laser fibers in microsurgical removal of acoustic neuromas (vestibular schwannomas). Clin Neurol Neurosurg 2016;145:35-40. [Crossref] [PubMed]
- Campero A, Martins C, Rhoton A Jr, et al. Dural landmark to locate the internal auditory canal in large and giant vestibular schwannomas: the Tübingen line. Neurosurgery 2011;69:ons99-102; discussion ons102. [PubMed]
- Wanibuchi M, Fukushima T, Friedman AH, et al. Hearing preservation surgery for vestibular schwannomas via the retrosigmoid transmeatal approach: surgical tips. Neurosurg Rev 2014;37:431-44; discussion 444. [Crossref] [PubMed]
- Mastronardi L, Di Scipio E, Cacciotti G, et al. Vestibular schwannoma and hearing preservation: Usefulness of level specific CE-Chirp ABR monitoring. A retrospective study on 25 cases with preoperative socially useful hearing. Clin Neurol Neurosurg 2018;165:108-15. [Crossref] [PubMed]
- Acioly MA, Liebsch M, de Aguiar PH, et al. Facial nerve monitoring during cerebellopontine angle and skull base tumor surgery: a systematic review from description to current success on function prediction. World Neurosurg 2013;80:e271-300. [Crossref] [PubMed]
- Corrivetti F, Cacciotti G, Giacobbo Scavo C, et al. Flexible Endoscopic-Assisted Microsurgical Radical Resection of Intracanalicular Vestibular Schwannomas by a Retrosigmoid Approach: Operative Technique. World Neurosurg 2018;115:229-33. [Crossref] [PubMed]
- Mastronardi L, Campione A. Diluted intracisternal papaverine for microvascular protection of cranial nerves during vestibular schwannoma and cerebello-pontine angle surgery. Commentary and review of the literature. J Clin Neurosci 2023;112:25-9. [Crossref] [PubMed]
- Kohno M, Sato H, Sora S, et al. Is an acoustic neuroma an epiarachnoid or subarachnoid tumor? Neurosurgery 2011;68:1006-16; discussion 1016-7. [Crossref] [PubMed]
- Sasaki T, Shono T, Hashiguchi K, et al. Histological considerations of the cleavage plane for preservation of facial and cochlear nerve functions in vestibular schwannoma surgery. J Neurosurg 2009;110:648-55. [Crossref] [PubMed]
- Nutik SL. Facial nerve outcome after acoustic neuroma surgery. Surg Neurol 1994;41:28-33. [Crossref] [PubMed]
- Troude L, Boucekine M, Montava M, et al. Predictive Factors of Early Postoperative and Long-Term Facial Nerve Function After Large Vestibular Schwannoma Surgery. World Neurosurg 2019;127:e599-608. [Crossref] [PubMed]
- Mastronardi L, Campione A, Boccacci F, et al. Koos grade IV vestibular schwannomas: considerations on a consecutive series of 60 cases-searching for the balance between preservation of function and maximal tumor removal. Neurosurg Rev 2021;44:3349-58. [Crossref] [PubMed]
- Cianfrone F, Cantore I, Roperto R, et al. Preoperative vestibular evoked myogenic potentials (VEMPs), caloric test, and pure tone audiometry to identify the vestibular nerve branch of schwannoma origin: preliminary results in a series of 26 cases. Neurosurg Rev 2022;45:3231-6. [Crossref] [PubMed]
- Nakatomi H, Jacob JT, Carlson ML, et al. Long-term risk of recurrence and regrowth after gross-total and subtotal resection of sporadic vestibular schwannoma. J Neurosurg 2017;133:1052-8. [Crossref] [PubMed]
- Kocharyan A, Daher GS, Curry SD, et al. Outcomes of Near-Total and Subtotal Resection of Sporadic Vestibular Schwannoma: A Systematic Review and Meta-Analysis. Otolaryngol Head Neck Surg 2024;171:642-57. [Crossref] [PubMed]
- Eiras J, Gomez J, Carcavilla L. Suboccipito-transmeatal microsurgical approach in giant acoustic neuromas. Results in 12 consecutive cases. Neurochirurgie 1984;30:17-24. [PubMed]
- Fundová P, Charabi S, Tos M, et al. Cystic vestibular schwannoma: surgical outcome. J Laryngol Otol 2000;114:935-9. [Crossref] [PubMed]
- Mehrotra N, Behari S, Pal L, et al. Giant vestibular schwannomas: focusing on the differences between the solid and the cystic variants. Br J Neurosurg 2008;22:550-6. [Crossref] [PubMed]
Cite this article as: Alomari AA, Campione A, Iavarone S, Alabdallat YJ, Alkhawaldeh IM, Mastronardi L. Optimizing retrosigmoid approach for giant vestibular schwannoma resection: strategies and advances in surgical technique. AME Surg J 2024;4:13.


