
Surgical strategies in the management of postoperative bronchopleural fistula: a narrative review
Introduction
Background
Bronchopleural fistula (BPF) is a multifaceted medical condition defined by the abnormal pathological communication between the bronchial tree and the pleural space, arising from the mainstem, lobar, or segmental bronchi (1). It disrupts the natural separation between these structures, leading to significant respiratory compromise due to pneumothorax and septic complications. BPF often manifests as a postoperative complication but may also arise from infections, malignancies, or trauma (2).
A hallmark of BPF is a development of pneumothorax with persistent air leak and its profound impact on respiratory mechanics. By creating a channel for direct entry of air and bronchial secretions into the pleural space, BPF results in pneumothorax, empyema, entrapped lung and sepsis (3). These complications impair oxygenation and lung function leading to severe respiratory distress. Clinically, patients with BPF may present with symptoms such as persistent air leak from chest drains, recurrent respiratory infections, persistent pleural effusions, and systemic signs of sepsis. Early recognition of these symptoms is crucial, as delayed intervention can result in significant complications, including multiorgan failure and death (4).
BPF most frequently occurs as a complication of anatomic lung resection procedures such as pneumonectomy or lobectomy. The reported incidence of BPF varies depending on the type and extent of the surgical intervention. Following a pneumonectomy, the incidence ranges from 4.5% to 20%, whereas after a lobectomy the incidence is lower, and is reported to occur in 0.5% to 1% of cases (5). BPF after pneumonectomy is often related to ischemia, high airway pressures, or bronchial stump dehiscence, whereas post-lobectomy BPF is significantly less common and frequently results from technical errors, incomplete bronchial closure, or impaired healing due to underlying conditions such as diabetes, chronic obstructive pulmonary disease (COPD), or corticosteroid use. The increased prevalence following pneumonectomy can be attributed to the larger bronchial stump, greater technical challenges involved in its closure, and large ex-vacuo pleural space. Additionally, right-sided pneumonectomies are more prone to BPF due to anatomical characteristics of the bronchial tree, including the lack of soft tissue coverage and the challenges associated with achieving a secure closure (6). The development of BPF in these contexts underscores the need for meticulous surgical technique and postoperative vigilance in high-risk patients. While routine bronchial stump buttressing with well-vascularized tissue has traditionally been advocated to reduce the risk of BPF, emerging evidence suggests that a selective approach may be more appropriate (7). Risk stratification based on factors such as prior radiation therapy, malnutrition, and high-dose corticosteroid use can help identify patients who would benefit most from reinforcement. Furthermore, the optimal choice of buttressing tissue remains an area of active investigation, with muscle flaps, pericardial fat, and omentum among the most commonly considered options.
BPF remains a challenging complication of thoracic surgery due to its high morbidity, complex pathophysiology, and varied clinical presentations. Multiple risk factors, including patient comorbidities, surgical technique, and postoperative complications, contribute to its development. The timing of BPF onset serves as a critical determinant in its classification and management, with acute, subacute, and chronic forms presenting distinct challenges.
Given its multifactorial nature, BPF management requires a multidisciplinary approach that integrates surgical, bronchoscopic, and supportive strategies tailored to the individual patient. The primary goals of treatment include closing the fistula with eliminating the air leak, resolving persistent pleural space, addressing infectious complications, and optimizing respiratory function (8). Supportive measures, such as nutritional optimization, respiratory therapy, and infection control, are fundamental to improving patient outcomes. The choice of therapeutic intervention depends on the size and location of the fistula, the timing of its presentation, and the patient’s overall health status (9).
Rationale and knowledge gap
BPF represents a challenging postoperative complication with significant morbidity and mortality, particularly following anatomic lung resections such as pneumonectomy or lobectomy. Mortality rates for BPF are 18.2% at 30 days and 22.7% at 90 days for acute and subacute cases, respectively, while chronic BPF tends to have a lower overall mortality rate (10-12). Despite advances in surgical techniques and perioperative care, the management of BPF remains complex due to its multifactorial etiology, varied clinical presentations, and high-risk anatomical and physiological implications. The literature highlights the association of BPF with complications such as pneumothorax, empyema, and sepsis, all of which contribute to impaired respiratory mechanics and systemic deterioration (2,3,6,13,14). While numerous studies have identified risk factors such as advanced age, comorbidities, and technical challenges associated with bronchial stump closure, gaps remain in understanding how to optimally prevent, diagnose, and manage BPF across different clinical scenarios (15-21).
The classification of BPF into acute, subacute, and chronic phases underscores the variability in its onset and presentation, yet there is limited consensus on tailored interventions for each stage. Furthermore, the selection of definitive therapeutic strategies—ranging from conservative measures to surgical interventions—depends on multiple factors, including the size, timing, and anatomical location of the fistula. Current approaches often rely on a multidisciplinary framework, but the efficacy of specific treatment modalities, including advanced bronchoscopic techniques, remains underexplored. Bridging these knowledge gaps is critical for improving patient outcomes and reducing the burden of this devastating condition.
While previous reviews have addressed various aspects of BPF, including surgical and bronchoscopic management, recent advancements in diagnostic modalities, endoscopic interventions, and bioengineered therapies have not been comprehensively analyzed within a structured, multidisciplinary framework. Additionally, there remains a need for an updated synthesis comparing treatment efficacy, patient selection criteria, and strategies for optimizing long-term outcomes. By bridging these knowledge gaps, this review aims to enhance clinical decision-making, refine treatment algorithms, and reduce the burden of this complex condition.
Objective
The objective of this study is to provide a comprehensive review of the current understanding of BPF, including its pathophysiology, risk factors, classification, and management strategies. By consolidating existing literature and clinical insights, this review aims to identify key knowledge gaps, evaluate the efficacy of diagnostic and therapeutic interventions, and propose evidence-based recommendations to guide clinical decision-making. Emphasizing both traditional and emerging therapies, this manuscript seeks to provide an updated perspective on multidisciplinary treatment approaches, ensuring alignment with recent advancements in thoracic surgery, interventional pulmonology, and regenerative medicine. Ultimately, the goal is to enhance prevention, facilitate timely diagnosis, and improve treatment strategies, thereby optimizing patient outcomes in this challenging clinical scenario. We present this manuscript in accordance with the Narrative Review reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-24-55/rc).
Methods
A comprehensive search of electronic databases, including PubMed, Scopus, Web of Science, Cochrane Library and Google Scholar, was conducted to identify peer-reviewed articles on BPF. The search strategy included a combination of medical subject headings (MeSH) and free-text terms, such as “bronchopleural fistula”, “postoperative complications”, “pneumonectomy”, “lobectomy”, “air leak”, “pneumothorax”, “bronchial stump closure”, and “management strategies”. Boolean operators (“AND”, “OR”) were employed to refine the search results and ensure comprehensive coverage. No language restrictions were applied, but priority was given to studies published in English.
Articles published between 2000 and 2024 were included, focusing on studies that provided insights into the etiology, risk factors, classification, diagnostic modalities, and management of BPF. Eligible studies included randomized controlled trials, observational studies, case series, systematic reviews, and expert opinion articles. Exclusion criteria included studies with incomplete data, non-human studies, and articles that were exclusively focused on unrelated conditions.
Key data points, including study design, patient population, risk factors, timing of BPF onset, diagnostic approaches, therapeutic interventions, and clinical outcomes, were extracted from the included articles. The extracted data were qualitatively synthesized to highlight current knowledge, identify gaps, and draw conclusions on the effectiveness of diagnostic and management strategies. Although formal quality assessment tools were not used due to the narrative nature of this review, the relevance and reliability of the included studies were evaluated by considering study design, sample size, and methodological rigor. Systematic reviews and high-quality observational studies were given greater weight in the synthesis of evidence (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 12 November 2024 |
| Databases and other sources searched | PubMed, Scopus, Web of Science, Cochrane Library and Google Scholar |
| Search terms used | Search terms included a combination of MeSH and free-text terms: “bronchopleural fistula”, “postoperative complications”, “pneumonectomy”, “lobectomy”, “air leak”, “pneumothorax”, “bronchial stump closure”, and “management strategies” |
| Timeframe | Studies published between 2000 and 2024 |
| Inclusion and exclusion criteria | Inclusion criteria: studies focusing on BPF, including pathophysiology, risk factors, classification, diagnostic approaches, and management strategies. Types included randomized controlled trials, observational studies, case series, systematic reviews, and expert opinions |
| Exclusion criteria: studies with incomplete data, non-human studies, and articles focusing on unrelated conditions. No language restrictions applied, but studies in English were prioritized | |
| Selection process | The selection was conducted independently by two reviewers. Titles and abstracts were screened initially, followed by a full-text review of potentially relevant articles. Discrepancies in selection were resolved through consensus discussions or by consulting a third reviewer |
| Any additional considerations, if applicable | Articles emphasizing innovative or multidisciplinary approaches to BPF management were prioritized. Studies focusing exclusively on pediatric populations or non-lung pathologies were excluded |
BPF, bronchopleural fistula.
Risk factors and diagnostic evaluation
A range of patient-related factors has been identified as contributing to the risk of developing BPF. These factors include advanced age, male gender, smoking, diabetes mellitus, COPD, and poor nutritional status, all of which can impair healing and increase susceptibility to complications (22). Additionally, systemic conditions such as prolonged corticosteroid use, or immunosuppression can compromise the structural integrity of the bronchial stump. Previous ipsilateral thoracotomy, extensive lymph node dissection, and radiation therapy further exacerbate the risk by introducing additional trauma or impairing tissue viability (21). Such multifactorial risks highlight the complexity of BPF as a postoperative complication and the importance of individualized patient assessment and management.
The timing of BPF onset varies widely and serves as a basis for its classification. Acute BPF typically occurs within the first 7 days postoperatively and is often due to mechanical failure of the bronchial stump closure (23). This early failure is characterized by the sudden onset of severe symptoms, including dyspnea, hypotension, subcutaneous emphysema, and expectoration of large volume secretions, i.e., pleural fluid. Imaging studies in these cases frequently reveal decreasing volume of pleural fluid after pneumonectomy, suggesting communication between the bronchial tree and the pleural cavity. Subacute BPF, occurring between 7 to 30 days postoperatively, is more insidious in its presentation, with symptoms such as fever, malaise, and respiratory distress. This delayed presentation is frequently associated with secondary infections, which complicate the clinical course (21). Chronic BPF, by contrast, develops more than 30 days after the surgery and is often linked to pleural fibrosis, granulomatous inflammation, infection and malignancy. These cases are marked by persistent respiratory symptoms such as cough, fatigue, and chest pain, and they often necessitate prolonged care (6).
The diagnostic evaluation of BPF requires a multifaceted approach that integrates clinical assessment, imaging studies, bronchoscopic examination, and supplementary diagnostic techniques. This comprehensive strategy is essential to confirm the presence and extent of the abnormal communication between the bronchial tree and the pleural cavity, ensuring timely and effective management. A thorough clinical assessment forms the foundation of BPF diagnosis. Patients often present with non-specific symptoms such as fever, cough, and hypoxemia, particularly when an infected pleural cavity is involved. While there are no laboratory tests specific to BPF, elevated inflammatory markers—erythrocyte sedimentation rate (ESR), also known as Biernacki’s reaction, and C-reactive protein (CRP), along with leukocytosis, are common findings in these patients (12). These abnormalities, when coupled with clinical signs, should heighten suspicion and prompt further investigation.
Radiological imaging is pivotal in diagnosing BPF, with chest computed tomography (CT) serving as the primary modality (24). CT scans provide detailed visualization of the pleural cavity and bronchial structures, making it possible to detect critical features indicative of BPF (Figure 1). These include persistent or increasing intrapleural air despite appropriately positioned chest tube, air-fluid levels that signify communication between the airways and pleural cavity, and changes in pleural effusion volumes or pneumothorax over time (Figure 2).
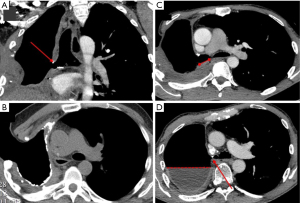

Advanced imaging techniques have further enhanced diagnostic accuracy for BPF. Three-dimensional spiral CT reconstruction improves visualization of the bronchial tree and surrounding structures, providing detailed insights that aid both diagnosis and treatment planning. CT bronchography, which involves injecting contrast medium in the bronchial tree, allows for highly precise visualization of the fistula and its anatomical relationships (25). While less commonly employed, radiolabeled aerosol inhalation has also been utilized for documenting BPFs; however, its use is limited by potential false positives and technical challenges.
Bronchoscopy is a cornerstone of BPF evaluation, allowing direct visualization of the bronchial stump and the airways to confirm the presence of the fistula (26). This procedure is particularly useful for localizing the site of air leakage, excluding other pathologies such as malignancy, and facilitating therapeutic interventions such as the application of endobronchial sealants or occlusion devices. In cases where the fistula is not readily visible, proximal balloon occlusion during bronchoscopy leading to cessation of air leakage, which helps identify smaller or more distal fistulas (6).
Supplementary diagnostic techniques are also valuable in specific context. The methylene blue test, which involves instilling the dye into the trachea and observing for leakage into the chest drainage system, is a straightforward and cost-effective method to confirm the presence of a fistula, albeit it is not specific in localization (9). However, this technique may miss multiple fistulas unless combined with other diagnostic approaches. Capnography, which measures end-tidal carbon dioxide levels during bronchial occlusion, provides a highly sensitive and specific method for localizing BPFs. Analyzing pleural air samples for oxygen and carbon dioxide levels can offer additional indirect evidence of communication with the bronchial tree (27). Ventilation imaging techniques, such as radionuclide studies, are occasionally employed for BPF localization but are less commonly used due to logistical challenges and the availability of more advanced imaging modalities (13). Contrast injection distal to occluded bronchus can help identify fistula localization.
Accurate and timely documentation and localization of BPF is critical to guide effective management and prevent progression to severe complications. The critical synthesis of clinical presentation, imaging results, bronchoscopic findings, and supplemental techniques ensures a thorough and accurate evaluation. Emerging diagnostic modalities, such as CT bronchography and three-dimensional imaging, further enhance the precision of BPF detection and characterization. These advancements, coupled with a multidisciplinary diagnostic approach, are essential for formulating an optimal treatment strategy tailored to each patient.
Management
The management of BPF requires a comprehensive and multidisciplinary strategy involving initial stabilization, supportive care, and interventions tailored to the specific characteristics of the fistula and the patient’s overall health. The primary objectives are to prevent severe complications such as aspiration, acute respiratory distress syndrome, and empyema while addressing the underlying etiology of the fistula. Treatment is highly individualized, based on multiple factors such as size, location, and timing of the fistula, as well as any associated pulmonary conditions. Acute BPF, which typically develops within the first 7 days postoperatively, often requires urgent surgical intervention due to the risk of rapid clinical deterioration. Immediate management focuses on stabilizing the patient through chest tube drainage, infection control, and ventilatory support, followed by definitive surgical repair. In cases where there is significant bronchial stump dehiscence or extensive necrosis, bronchial stump revision, reinforcement with vascularized tissue flaps, or even completion pneumonectomy may be necessary to achieve adequate closure and prevent further air leakage. In contrast, chronic BPF, which develops weeks to months after surgery, is often managed in a staged manner, integrating prolonged pleural drainage, infection control, and, where feasible, bronchoscopic interventions such as endobronchial valve (EBV) placement or sealant application. For larger, persistent fistulas, surgical options such as muscle flap transposition, omental pedicle reinforcement, and thoracoplasty may be required to obliterate the pleural space and ensure long-term closure. A multidisciplinary approach is essential in both acute and chronic cases to tailor management strategies based on patient comorbidities, fistula characteristics, and overall prognosis.
Management typically begins with the stabilization of the patient, which includes initial pleural drainage via a large bore chest tube, facilitating drainage of empyema, and preventing aspiration of the infected material into the contralateral lung (12). Proper positioning, such as placing the affected lung in a dependent position, enhances pleural drainage and reduces the risk of aspiration. In mechanically ventilated patients, careful adjustments to minimize mean airway pressure and reduce tidal volumes are essential to decrease air leakage through the fistula and maintain adequate oxygenation (28). Chest tube thoracostomy serves as the cornerstone of early intervention, ensuring effective pleural drainage and aiding in infection control. Broad-spectrum intravenous antibiotics are initiated promptly to cover gram-positive, gram-negative anaerobic and fungal organisms, with the regimen later tailored based on culture results (29). Supportive care aims to optimize the patient’s respiratory status and overall condition.
Non-invasive management is often the first-line approach for small fistulas (less than 8 mm) or for patients who are poor surgical candidates (22). This approach is particularly suitable for fistulas ≤5 mm, as spontaneous closure is more likely, whereas larger fistulas (5–8 mm) may require adjunctive bronchoscopic interventions. Conservative measures focus on ensuring effective drainage of air and fluid, preventing infection, and maintaining respiratory function. While mechanical ventilation may be necessary, it can increase the risk of BPF formation; therefore, low tidal volumes, decreased peak respiratory pressures, reduced respiratory rates, and shortened inspiratory times are recommended to minimize airway pressures (12). Although conservative treatment alone may not fully close BPFs, it can facilitate partial closure, particularly in smaller fistulas, when combined with bronchoscopic interventions. These measures, coupled with diligent pleural drainage and fluid balance maintenance, can significantly improve outcomes (30).
For acute BPFs, surgical intervention may involve surgical suturing of the bronchial stump with vascularized flap coverage to enhance healing and prevent recurrence. Video-assisted or robotic interventions may be employed for these repairs, though thoracotomy may still be required (14). Techniques, such as muscle or omental flap coverage or thoracoplasty may be used to fill or obliterate the pleural space or reinforce the bronchial stump (8). When postoperative pleural effusions (PPEs) accompany BPF, surgical management often involves pleural debridement, revision of the bronchial stump, and reinforcement with vascularized tissue (9).
BPFs that occur weeks or even years after pneumonectomy often require a staged approach to management (8). Small chronic fistulas may be managed non-operatively, but larger ones typically necessitate surgical intervention to reduce aspiration risk and restore pulmonary integrity. Postoperative complications, including recurrent PPE and BPF, often require re-do thoracotomy for debridement and repair with vascularized tissue reinforcement. For these delayed cases, the integrity of the surrounding tissue may dictate the choice of intervention, ranging from conservative management to advanced reconstructive techniques.
For patients not suitable for surgery, bronchoscopic techniques can offer a minimally invasive alternative (31). These approaches include the application of sealants, stents, or other occlusive materials to close small, distal fistulas. Success rates for bronchoscopic management range from 71% to 92%, particularly for fistulas smaller than 6 mm (30). In cases where bronchoscopic interventions are insufficient, they can serve as a bridge to more definitive surgical procedures.
Endoscopic methods for BPF management
The management of BPF has seen remarkable advancements with the evolution of bronchoscopic techniques. These minimally invasive methods have revolutionized treatment, offering promising outcomes and serving as critical alternatives to surgical interventions, particularly for patients with significant surgical risk. Success rates for bronchoscopic management range from 30% to 96%, depending on the size and complexity of the fistula, as well as the method employed (32). These approaches are increasingly tailored to individual patient needs, incorporating a range of technologies and substances designed to promote closure, reduce air leaks, and enhance tissue healing.
Techniques for small fistulas (less than 3 mm)
For small BPFs, minimally invasive techniques such as ethanol injections, fibrin glues, and silver nitrate cautery have demonstrated effectiveness. Ethanol injection, as pioneered by Takaoka et al., involves administering absolute ethanol into the submucosal layer of the fistula (33). This induces localized edema and fibrous scarring, effectively sealing defects smaller than 1 mm. While this approach is highly effective for tiny fistulas, larger ones may require additional measures. Sclerosing agents like silver nitrate and polidocanol have been employed to induce localized fibrosis. Stratakos et al. reported an 81.8% success rate with silver nitrate, while Varoli et al. achieved a 65% closure rate using polidocanol for fistulas up to 10 mm in diameter (23,34). Although polidocanol has been reported to be effective for BPFs <10 mm, limited evidence supports its primary application in small fistulas.
Fibrin-based glues, such as Tisseel and Evicel, are widely utilized for their ability to mechanically seal fistulas while promoting inflammation and fibrosis to encourage long-term closure (35). These glues are typically applied via double-lumen catheters and form a durable clot at the defect site. However, multiple applications are often necessary, particularly for large size fistulas.
Intermediate fistulas (3–5 mm)
Bronchoscopic management of fistulas in the 3–5 mm range often necessitates combining multiple techniques to achieve optimal outcomes. Marwah et al. highlighted the use of endobronchial coils, atrial septal occluder devices, and tissue adhesives such as Bioglue (36). Endobronchial coils, deployed via fiberoptic bronchoscope, are anchored to the fistula site and supplemented with Bioglue to seal small gaps. Despite their utility, these techniques can be less effective, with a failure rate of up to 40% for fistulas larger than 4 mm. Zhu et al. proposed a three-step protocol that combines sealants, Neoveil patch (bioabsorbable reinforcement felt composed of polyglycolic acid fibers; Gunze Co., Tokyo, Japan), and reinforced sutures for fistulas up to 5 mm (37). This innovative approach achieved an impressive 93.6% success rate, underscoring the importance of customizing treatments to the size and characteristics of the defect.
Techniques for larger fistulas (greater than 5 mm)
Larger BPFs present unique challenges that often necessitate mechanical occluders or stents. Amplatzer septal occluders (ASOs), originally designed for cardiac defects, have been adapted for bronchoscopic use, providing a mechanical seal while promoting granulation tissue formation (31,38). These devices boast success rates as high as 96%, although complications such as migration and infection can occur.
Self-expandable metallic stents (SEMS) and silicone stents play distinct roles in managing large or centrally located BPFs, with significant differences based on their design and coverage (39). Fully covered stents, whether metallic or silicone, offer the most promise in sealing BPFs effectively, as their complete coverage prevents air and fluid leakage, achieving the primary goal of fistula closure. However, their main drawback is the increased risk of migration due to reduced friction with the airway wall. Partially covered stents, while offering better anchorage and stability through uncovered ends, are less effective for complete fistula management as leakage can still occur, and they carry the risk of granulation tissue formation and epithelial overgrowth. Uncovered stents, designed primarily for airway stabilization rather than sealing, are largely unsuitable for BPFs, as they permit air and fluid passage and are associated with significant complications such as granulation tissue formation and difficult removal. Silicone stents, such as Dumon stents, though less commonly used for BPFs, provide a fully covered option with excellent biocompatibility and a lower risk of granulation tissue formation, but their lower radial force and higher migration risk limit their utility in complex cases. Zeng et al. reported an initial success rate of 94.1% with silicone stents, which declined to 76.5% over a median follow-up period of 107 days (range, 5–431 days) (40). While metal stents are durable, long-term use can lead to complications, including airway wall erosion and infection. Overall, fully covered stents remain the most viable option for managing BPFs, as they ensure effective sealing while minimizing complications.
Advanced devices and emerging therapies
EBVs, such as Zephyr and Spiration valves, are innovative devices initially designed for emphysema and prolonged air leaks but have demonstrated success rates exceeding 70% for BPFs (26). These one-way valves allow air and fluid escape from the affected segment while preventing air entry for ventilation. Despite their effectiveness, high cost and steep learning curve limit widespread adoption of many of these devices, including stents and Amplatzer occluders.
The endobronchial Watanabe spigot (EWS), a silicone bronchial plug available in various diameters, has been particularly effective for pneumothorax and postoperative air leaks. A retrospective study by Himeji et al. reported an 85.7% success rate with EWS, although migration remains a common complication (41).
Emerging therapies include mesenchymal stem cells (MSCs) and 3D-printed stents. MSCs have shown remarkable promise in early studies, achieving 100% success rates in post-pneumonectomy BPFs (42). These cells promote regeneration and tissue repair through their differentiation capabilities and low immunogenicity. 3D-printed stents represent another frontier in BPF management, offering the ability to customize devices to individual airway anatomy. These stents are quickly manufactured, reducing the time to intervention, and are expected to become integral to BPF treatment.
Challenges and future directions
Bronchoscopic management of BPFs offers significant advantages, including reduced morbidity, shorter hospital stays, and avoidance of invasive surgery. However, these interventions require careful consideration of patient-specific factors, including tissue necrosis, fistula size, and comorbid conditions. The complexity of these procedures underscores the importance of experienced practitioners and multidisciplinary collaboration. Continued innovation in device design and treatment strategies holds promise for improving outcomes. Advancements in biocompatible adhesives, sheath-free occluders, and regenerative therapies like stem cell applications are poised to transform the field. Further comparative studies and long-term follow-up data are essential to refine existing techniques and evaluate the efficacy of emerging approaches. With these advancements, bronchoscopic management is increasingly recognized as a cornerstone in the treatment of BPFs, offering hope for improved outcomes and quality of life for affected patients.
Surgical methods for BPF management
The management of large BPF often requires surgical intervention, particularly when endoscopic techniques are insufficient. Surgical closure remains challenging due to the frequent presence of empyema. Advances in surgical techniques have significantly improved outcomes, offering effective solutions for even the most complex cases.
Historical perspectives and evolution of techniques
Historically, surgical approaches to BPF focused on managing sepsis and draining the pleural space. One of the earliest techniques, the open window thoracostomy (OWT), was introduced by Leo Eloesser in 1935 (43). While effective in controlling infection, OWT often left patients with permanent chest wall defects, and committed to extended if not lifelong wound care. In the 1960s, Clagett and Geraci introduced a modified OWT combined with antibiotic irrigation and eventual closure of the thoracic cavity, a pivotal advancement in BPF management (44). Thoracoplasty, a surgical technique involving removal of portions of the rib cage, is an established approach for obliterating residual pleural spaces in the management of BPFs (9). This procedure is particularly effective in cases where persistent infection, empyema, or inadequate lung expansion prevents natural space closure. By collapsing the chest wall into the pleural cavity, thoracoplasty reduces the dead space, promotes apposition of tissues, and aids in fistula closure while minimizing the risk of recurrent infection. Soft tissue transposition for the obliteration of the pleural cavity and particularly muscle flaps, as described by Pairolero and colleagues, added another dimension, reducing recurrence rates and improving bronchial stump reinforcement (45). Modern modifications, including muscle flap transposition or omental pedicles, are often incorporated to enhance the space obliteration and support tissue healing (46). Although thoracoplasty is considered a last-resort intervention due to its significant impact on chest wall mechanics and body contour deformity, it remains a lifesaving option for patients with refractory BPFs. This technique continues to demonstrate success in highly selected cases, particularly when combined with tailored antimicrobial therapy and supportive care.
Modern surgical strategies
Muscle flap transposition
Muscle flap transposition is now a cornerstone of BPF management, particularly for closing large fistulas and obliterating residual pleural spaces (47). This approach utilizes vascularized muscle tissue to reinforce the bronchial stump, enhance healing, and reduce recurrence risks (48). Common muscle flaps include:
- Latissimus dorsi: a versatile option due to its bulk and especially for apical and mid-thoracic defects (Figure 3) (49,50).
- Pectoralis major: effective for smaller defects and sternal infections, often used in combination with other flaps (51,52).
- Serratus anterior: ideal for smaller spaces (53-55).
- Rectus abdominis: ideal for defects in basal pleural zones (56-58).

Omental flaps, transferred through transdiaphragmatic incisions, are also employed due their rich vascular supply and ability to obliterate dead space. These flaps are particularly useful for patients with extensive infections or large residual cavity in the basilar aspect of the pleural space (Figures 4,5) (8,59,60).


OWT
OWT remains a lifesaving intervention for patients with complex BPFs and severe pleural infections. This procedure involves resecting ribs to create a chest wall window, enabling continuous drainage and debridement (8). While traditionally a definitive solution, OWT is now often employed as a temporary measure before soft tissue flap transposition and cavity closure (46). Pulmonary artery fistula (PAF) is a rare but life-threatening complication that can develop following OWT for BPF. This condition involves an abnormal connection between the pulmonary artery and adjacent structures, such as the bronchial tree or pleural space, leading to catastrophic complications, including massive hemoptysis and severe infection (61). While the exact incidence of PAF in the setting of BPF remains undefined, its occurrence is associated with high morbidity and mortality (62). Early recognition through imaging and bronchoscopy, coupled with a swift multidisciplinary approach, is critical for optimizing patient outcomes. Given the substantial risk of fatal hemorrhage, surgical intervention is typically required, as conservative management alone is rarely sufficient.
Recent advancements, such as negative pressure wound therapy, have enhanced OWT by promoting granulation tissue formation, reducing infection, and accelerating recovery (63,64).
Surgical approach: transpleural vs. transsternal
The transpleural approach is the most commonly used technique for BPF closure. This method involves direct dissection of the bronchial stump from surrounding tissues with minimal devascularization. Sutures or stapling devices are often used, with muscle or omental flaps providing additional reinforcement. For cases where transpleural access is inappropriate, such as short or necrotic bronchial stumps or carinal involvement, a transsternal transpericardial approach is an effective alternative (1,6). This approach via sternotomy and pericardium, leads directly to the distal trachea and carina, allowing precise repair of complex bronchial defects while avoiding infected and fibrotic planes of the thoracic cavity. When combined with robust infection control, this method yields high success rates and reduced recurrence.
Minimally invasive approach
Minimally invasive techniques, such as video-assisted thoracic surgery (VATS), are increasingly utilized for BPFs and early-stage empyema (50,65). Minimally invasive interventions enable debridement, drainage and decortication with minimal morbidity, making it an ideal option for well-contained fistulas. While not suitable for large or chronic BPFs, requiring soft tissue transposition, these approaches often serve as a precursor to more invasive procedures.
Combined and staged approaches
In complex cases involving large, infected pleural space, a combination of multiple technologies may be required. For example, tailored thoracoplasty can be paired with muscle and omental flap transposition to obliterate large spaces (46). Recent studies support the use of accelerated treatment protocols, which involve repeated debridement under anesthesia, followed by antibiotic irrigation and early closure. These protocols have demonstrated excellent outcomes, reduced hospitalization times and improving fistula closure rates (66).
Innovations and future directions
Recent advancements in biomaterials and surgical techniques have opened new avenues for BPF management. Bioadhesive plugs and tissue-engineered scaffolds are being explored as alternatives to traditional surgical methods (67,68). These innovations aim to provide less invasive, more effective solutions for fistula closure while minimizing patient morbidity. Furthermore, robotic-assisted thoracic surgery (RATS) offers enhanced precision and access, particularly for complex reconstructions and delicate mediastinal dissection (69). As technology continues to evolve, the integration of these tools into standard surgical practice may further improve outcomes for patients with BPF.
Surgical methods remain a cornerstone in the management of large and complex BPFs. While traditional techniques like omental and muscle flap transposition and OWT continue to play vital roles, advancements in minimally invasive surgery, biomaterials, and robotic technology are reshaping the field. The integration of innovative approaches promises improved outcomes, shorter recovery times, and reduced recurrence, making surgical management an ever-evolving domain in BPF care.
Conclusions
BPFs remain one of the most challenging and feared complications in thoracic surgery, lacking a definitive gold standard for treatment. The wide array of management strategies, ranging from advanced bronchoscopic techniques employing various agents to surgical interventions using muscle or omental flaps, reflects the complexity and individualized nature of BPF care. While many studies demonstrate promising results, their generalizability is often constrained by small sample sizes and the heterogeneity of patient populations.
Given the high morbidity and mortality associated with BPFs, there is an urgent need for larger, well-designed multi-institutional studies to compare the effectiveness of these approaches, optimize treatment protocols, and reduce complications. Additionally, advancements in minimally invasive technologies, biomaterials, and regenerative therapies hold significant potential for improving outcomes and expanding the arsenal of therapeutic options.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-24-55/rc
Peer Review File: Available at https://asj.amegroups.com/article/view/10.21037/asj-24-55/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-24-55/coif). A.I.G. serves as an unpaid editorial board member of AME Surgical Journal from February 2024 to January 2026. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration and its subsequent amendments. Written informed consent was obtained from the patient for the publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Petrov RV, Bakhos CT, Abbas AE. Carinal resection. Shanghai Chest 2018;2:84. [Crossref] [PubMed]
- Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest 2005;128:3955-65. [Crossref] [PubMed]
- Yang YH, Park SY, Kim HE, et al. Postoperative bronchopleural fistula repair: Surgical outcomes and adverse factors for its success. Thorac Cancer 2022;13:1401-5. [Crossref] [PubMed]
- Galetta D, Spaggiari L. Video-Thoracoscopic Management of Postpneumonectomy Empyema. Thorac Cardiovasc Surg 2018;66:701-6. [Crossref] [PubMed]
- Nagahiro I, Aoe M, Sano Y, et al. Bronchopleural fistula after lobectomy for lung cancer. Asian Cardiovasc Thorac Ann 2007;15:45-8. [Crossref] [PubMed]
- Gritsiuta AI, Bakhos CT, Abbas AE, et al. Transsternal Approach for Broncho-Pleural Fistula Closure After Right Pneumonectomy. Cureus 2023;15:e50397. [Crossref] [PubMed]
- Skrzypczak P, Roszak M, Kasprzyk M, et al. The technique of stump closure has no impact on post-pneumonectomy bronchopleural fistula in the non-small cell lung cancer-a cross-sectional study. J Thorac Dis 2022;14:3343-51. [Crossref] [PubMed]
- Gritsiuta AY, Eguchi T, Jones DR, et al. A Stepwise Approach for Postlobectomy Bronchopleural Fistula. Oper Tech Thorac Cardiovasc Surg 2020;25:85-104. [Crossref] [PubMed]
- Bribriesco A, Patterson GA. Management of Postpneumonectomy Bronchopleural Fistula: From Thoracoplasty to Transsternal Closure. Thorac Surg Clin 2018;28:323-35. [Crossref] [PubMed]
- Gursoy S, Yazgan S, Ucvet A, et al. Postpneumonectomy bronchopleural fistula in non-small cell lung cancer patients: incidence, survival, mortality, and treatment analysis. Surg Today 2018;48:695-702. [Crossref] [PubMed]
- Wang Y, Zhu M, Pan Y, et al. Long-term follow up and comparison between conservative and interventional therapy in postoperative bronchopleural fistula-a cohort study. J Thorac Dis 2023;15:1210-6. [Crossref] [PubMed]
- Mazzella A, Casiraghi M, Uslenghi C, et al. Bronchopleural Fistula after Lobectomy for Lung Cancer: How to Manage This Life-Threatening Complication Using Both Old and Innovative Solutions. Cancers (Basel) 2024;16:1146. [Crossref] [PubMed]
- Grotberg JC, Hyzy RC, De Cardenas J, et al. Bronchopleural Fistula in the Mechanically Ventilated Patient: A Concise Review. Crit Care Med 2021;49:292-301. [Crossref] [PubMed]
- Gossot D, Stern JB, Galetta D, et al. Thoracoscopic management of postpneumonectomy empyema. Ann Thorac Surg 2004;78:273-6. [Crossref] [PubMed]
- Sonobe M, Nakagawa M, Ichinose M, et al. Analysis of risk factors in bronchopleural fistula after pulmonary resection for primary lung cancer. Eur J Cardiothorac Surg 2000;18:519-23. [Crossref] [PubMed]
- Matsunaga T, Suzuki K, Hattori A, et al. Risk factors for bronchopleural fistula based on surgical procedure and sex in 4794 consecutive patients undergoing anatomical pulmonary resection. Surg Today 2024;54:617-26. [Crossref] [PubMed]
- Mammana M, Marulli G, Zuin A, et al. Postpneumonectomy bronchopleural fistula: analysis of risk factors and the role of bronchial stump coverage. Surg Today 2020;50:114-22. [Crossref] [PubMed]
- Nachira D, Chiappetta M, Fuso L, et al. Analysis of risk factors in the development of bronchopleural fistula after major anatomic lung resection: experience of a single centre. ANZ J Surg 2018;88:322-6. [Crossref] [PubMed]
- Algar FJ, Alvarez A, Aranda JL, et al. Prediction of early bronchopleural fistula after pneumonectomy: a multivariate analysis. Ann Thorac Surg 2001;72:1662-7. [Crossref] [PubMed]
- Ichinose J, Hashimoto K, Matsuura Y, et al. Risk factors for bronchopleural fistula after lobectomy for lung cancer. J Thorac Dis 2023;15:3330-8. [Crossref] [PubMed]
- Hu XF, Duan L, Jiang GN, et al. A clinical risk model for the evaluation of bronchopleural fistula in non-small cell lung cancer after pneumonectomy. Ann Thorac Surg 2013;96:419-24. [Crossref] [PubMed]
- Skrzypczak PJ, Kasprzyk M, Piwkowski C. The review of the management and prevention methods of bronchopleural fistula in thoracic surgery. J Thorac Dis 2023;15:5268-71. [Crossref] [PubMed]
- Varoli F, Roviaro G, Grignani F, et al. Endoscopic treatment of bronchopleural fistulas. Ann Thorac Surg 1998;65:807-9. [Crossref] [PubMed]
- Kim EA, Lee KS, Shim YM, et al. Radiographic and CT findings in complications following pulmonary resection. Radiographics 2002;22:67-86. [Crossref] [PubMed]
- Sarkar P, Patel N, Chusid J, et al. The role of computed tomography bronchography in the management of bronchopleural fistulas. J Thorac Imaging 2010;25:W10-3. [Crossref] [PubMed]
- Jin L, Li Y. Bronchoscopic interventions for bronchopleural fistulas. Ther Adv Respir Dis 2023;17:17534666231164541. [Crossref] [PubMed]
- Hull MS, Nader D, Fullingim J. The diagnosis and localization of a bronchopleural fistula using single proton emission computer tomography (SPECT) imaging. Chest 2008;134:18C. [Crossref]
- Mao R, Ying PQ, Xie D, et al. Conservative management of empyema-complicated post-lobectomy bronchopleural fistulas: experience of consecutive 13 cases in 9 years. J Thorac Dis 2016;8:1577-86. [Crossref] [PubMed]
- Clark JM, Cooke DT, Brown LM. Management of Complications After Lung Resection: Prolonged Air Leak and Bronchopleural Fistula. Thorac Surg Clin 2020;30:347-58. [Crossref] [PubMed]
- Cardillo G, Carbone L, Carleo F, et al. The Rationale for Treatment of Postresectional Bronchopleural Fistula: Analysis of 52 Patients. Ann Thorac Surg 2015;100:251-7. [Crossref] [PubMed]
- Abbas AE. Commentary: Endoscopic device closure to fix the dam problem of postpneumonectomy bronchopleural fistula. JTCVS Tech 2020;4:349-50. [Crossref] [PubMed]
- West D, Togo A, Kirk AJ. Are bronchoscopic approaches to post-pneumonectomy bronchopleural fistula an effective alternative to repeat thoracotomy? Interact Cardiovasc Thorac Surg 2007;6:547-50. [Crossref] [PubMed]
- Takaoka K, Inoue S, Ohira S. Central bronchopleural fistulas closed by bronchoscopic injection of absolute ethanol. Chest 2002;122:374-8. [Crossref] [PubMed]
- Stratakos G, Zuccatosta L, Porfyridis I, et al. Silver nitrate through flexible bronchoscope in the treatment of bronchopleural fistulae. J Thorac Cardiovasc Surg 2009;138:603-7. [Crossref] [PubMed]
- Boudaya MS, Smadhi H, Zribi H, et al. Conservative management of postoperative bronchopleural fistulas. J Thorac Cardiovasc Surg 2013;146:575-9. [Crossref] [PubMed]
- Marwah V, Katoch CDS, Kumar K, et al. Bronchoscopic device closure of postoperative bronchopleural fistulae: Novel devices and innovative techniques. Lung India 2020;37:107-13. [Crossref] [PubMed]
- Zhu M, Yang Y, Shi Y, et al. A treatment protocol for chronic post-pneumonectomy empyema associated with bronchopleural fistula: A single-centre retrospective study. Int Wound J 2023;20:725-31. [Crossref] [PubMed]
- Fruchter O, Bruckheimer E, Raviv Y, et al. Endobronchial closure of bronchopleural fistulas with Amplatzer vascular plug. Eur J Cardiothorac Surg 2012;41:46-9. [PubMed]
- Ayub A, Al-Ayoubi AM, Bhora FY. Stents for airway strictures: selection and results. J Thorac Dis 2017;9:S116-21. [Crossref] [PubMed]
- Zeng J, Wu X, Chen Z, et al. Modified silicone stent for the treatment of post-surgical bronchopleural fistula: a clinical observation of 17 cases. BMC Pulm Med 2021;21:10. [Crossref] [PubMed]
- Himeji D, Tanaka GI, Fukuyama C, et al. Clinical Evaluation of Endoscopic Bronchial Occlusion with an Endobronchial Watanabe Spigot for the Management of Intractable Pneumothorax, Pyothorax with Bronchial Fistula, and Postoperative Air Leakage. Intern Med 2020;59:1835-9. [Crossref] [PubMed]
- Bottoni E, Banzatti BP, Novellis P, et al. Endoscopic Lipofilling for the Treatment of Bronchopleural Fistulas After Anatomic Lung Resection. Ann Thorac Surg 2021;111:e143-5. [Crossref] [PubMed]
- Allen MS. 50th anniversary landmark commentary on Eloesser L. Of an operation for tuberculous empyema. Ann Thorac Surg 1969;8:355-7. Ann Thorac Surg 2015;99:753. [Crossref] [PubMed]
- Clagett OT, Geraci JE. A procedure for the management of postpneumonectomy empyema. J Thorac Cardiovasc Surg 1963;45:141-5. [Crossref] [PubMed]
- Pairolero PC, Arnold PG, Trastek VF, et al. Postpneumonectomy empyema. The role of intrathoracic muscle transposition. J Thorac Cardiovasc Surg 1990;99:958-66; discussion 966-8. [Crossref] [PubMed]
- Shen KR, Bribriesco A, Crabtree T, et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg 2017;153:e129-46. [Crossref] [PubMed]
- Asaad M, Van Handel A, Akhavan AA, et al. Muscle Flap Transposition for the Management of Intrathoracic Fistulas. Plast Reconstr Surg 2020;145:829e-38e. [Crossref] [PubMed]
- Lu C, Feng Z, Ge D, et al. Pedicle muscle flap transposition for chronic empyema with persistent bronchopleural fistula: experience of a single clinical center in China. Surg Today 2016;46:1132-7. [Crossref] [PubMed]
- He Z, Shen L, Xu W, et al. Effective treatment of bronchopleural fistula with empyema by pedicled latissimus dorsi muscle flap transfer: Two case report. Medicine (Baltimore) 2020;99:e22485. [Crossref] [PubMed]
- Wolter A, Scholz T, Diedrichson J, et al. Bronchopleural fistula after pneumonectomy: interdisciplinary surgical closure by an ipsilateral pedicled latissimus dorsi flap supported by video-assisted thoracoscopy. J Plast Reconstr Aesthet Surg 2013;66:1600-3. [Crossref] [PubMed]
- Turk AE, Karanas YL, Cannon W, et al. Staged closure of complicated bronchopleural fistulas. Ann Plast Surg 2000;45:560-4. [Crossref] [PubMed]
- Ridgway E, DeCamp M, Morris D. Bronchopleural fistula repair using combined breast parenchymal and pectoralis major musculocutaneous flap. Ann Thorac Surg 2008;86:1022-5. [Crossref] [PubMed]
- Jester I, Nijran A, Singh M, et al. Surgical management of bronchopleural fistula in pediatric empyema and necrotizing pneumonia: efficacy of the serratus anterior muscle digitation flap. J Pediatr Surg 2012;47:1358-62. [Crossref] [PubMed]
- Park JS, Eom JS, Choi SH, et al. Use of a serratus anterior musculocutaneous flap for surgical obliteration of a bronchopleural fistula. Interact Cardiovasc Thorac Surg 2015;20:569-74. [Crossref] [PubMed]
- Groth SS, Whitson BA, D'Cunha J, et al. Serratus anterior transposition muscle flaps for bronchial coverage: technique and functional outcomes. Ann Thorac Surg 2009;88:2044-6. [Crossref] [PubMed]
- Brennan PG, Hsu DS, Banks KC, et al. Vertical rectus abdominis myocutaneous free flap repair of post-pneumonectomy bronchopleural fistula: a case report. AME Case Rep 2022;6:33. [Crossref] [PubMed]
- Huang JW, Lin YY, Wu NY, et al. Transverse rectus abdominis myocutaneous flap for postpneumonectomy bronchopleural fistula: A case report. Medicine (Baltimore) 2017;96:e6688. [Crossref] [PubMed]
- Jiang L, Jiang GN, He WX, et al. Free rectus abdominis musculocutaneous flap for chronic postoperative empyema. Ann Thorac Surg 2008;85:2147-9. [Crossref] [PubMed]
- Uchibori A, Okada S, Takeda-Miyata N, et al. Omental Flap for Bronchopleural Fistula After Pneumonectomy and Aorta Replacement. Ann Thorac Surg 2020;109:e349-51. [Crossref] [PubMed]
- D'Andrilli A, Ibrahim M, Andreetti C, et al. Transdiaphragmatic harvesting of the omentum through thoracotomy for bronchial stump reinforcement. Ann Thorac Surg 2009;88:212-5. [Crossref] [PubMed]
- Miyata R, Sonobe M, Yamawaki S, et al. External fistulous wound with Pseudomonas aeruginosa infection and massive bleeding following rupture of pulmonary suppuration. Interact Cardiovasc Thorac Surg 2012;14:903-5. [Crossref] [PubMed]
- Abe J, Hasumi T, Takahashi S, et al. Fatal broncho-pulmonary artery fistula after lobectomy for lung cancer†. J Surg Case Rep 2015;2015:rjv110. [Crossref] [PubMed]
- Laperuta P, Napolitano F, Vatrella A, et al. Post-pneumonectomy broncho-pleural fistula successfully closed by open-window thoracostomy associated with V.A.C. therapy. Int J Surg 2014;12:S17-9. [Crossref] [PubMed]
- Passera E, Guanella G, Meroni A, et al. Amplatzer device and vacuum-assisted closure therapy to treat a thoracic empyema with bronchopleural fistula. Ann Thorac Surg 2011;92:e23-5. [Crossref] [PubMed]
- Sagawa M, Sugita M, Takeda Y, et al. Video-assisted bronchial stump reinforcement with an intercostal muscle flap. Ann Thorac Surg 2004;78:2165-6. [Crossref] [PubMed]
- Schneiter D, Grodzki T, Lardinois D, et al. Accelerated treatment of postpneumonectomy empyema: a binational long-term study. J Thorac Cardiovasc Surg 2008;136:179-85. [Crossref] [PubMed]
- Ueda Y, Somamoto S, Kawabata S, et al. Development of a Novel Material to Promote Wound Healing at Bronchial Defects. Ann Thorac Surg 2023;116:239-45. [Crossref] [PubMed]
- Moriyama M, Matsumoto K, Taniguchi D, et al. Successful use of bio plugs for delayed bronchial closure after pneumonectomy in experimental settings. Interact Cardiovasc Thorac Surg 2022;34:660-7. [Crossref] [PubMed]
- Cohen BD, Marshall MB. Robotic-assisted tracheobronchial surgery. J Thorac Dis 2020;12:6173-8. [Crossref] [PubMed]
Cite this article as: Gritsiuta AI, Stovall A, Petrov RV. Surgical strategies in the management of postoperative bronchopleural fistula: a narrative review. AME Surg J 2025;5:10.


